Talanta ( IF 6.1 ) Pub Date : 2020-10-12 , DOI: 10.1016/j.talanta.2020.121711 Rohit Budhraja , Shubhangi Karande , Chang Ding , Maria K. Ullrich , Stephan Wagner , Thorsten Reemtsma , Lorenz Adrian
|
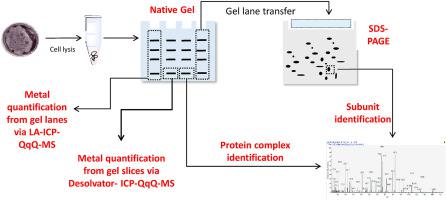
Membrane-bound metalloproteins are the basis of biological energy conservation via respiratory processes, however, their biochemical characterization is difficult. Here, we followed a gel-based proteomics and metallomics approach to identify membrane-associated metalloproteins in the anaerobic ammonium-oxidizing “Candidatus Kuenenia stuttgartiensis” strain CSTR1. Membrane-associated protein complexes were separated by two dimensional Blue Native/SDS gel electrophoresis and subunits were identified by mass spectrometry; protein-bound metal ions were quantified from the gel by connecting either a desolvating nebulizer system or laser ablation to inductively coupled plasma triple quadrupole mass spectrometry (ICP-QqQ-MS). We identified most protein complexes predicted to be involved in anaerobic ammonium oxidation and carbon fixation. The ICP-QqQ-MS data showed the presence of Fe and Zn in a wide range of high molecular weight protein complexes (230–800 kDa). Mo was prominently found in gel slices with proteins of a size of 500–650 kDa, whereas Ni was only found using the desolvating nebulizer system in the protein range of 350–500 kDa. The detected protein complexes and their metal content were consistent with genome annotations. Gel-based metalloproteomics is a sensitive and reliable approach for the characterization of metalloproteins and could be used to characterize many multimeric metalloprotein complexes in biological systems.
中文翻译:

在厌氧性氨氧化细菌“膜结合的金属蛋白的表征暂定Kuenenia stuttgartiensis”应变CSTR1
膜结合的金属蛋白是通过呼吸过程进行生物节能的基础,但是,其生化特性很难鉴定。在这里,我们遵循了基于凝胶的蛋白质组学和金属组学方法,以鉴定厌氧铵氧化“念珠菌”中与膜相关的金属蛋白。斯图加特犬(Kuenenia stuttgartiensis)菌株CSTR1。通过二维Blue Native / SDS凝胶电泳分离膜相关蛋白复合物,并通过质谱法鉴定亚基。通过将去溶剂化雾化器系统或激光烧蚀连接到电感耦合等离子体三重四极杆质谱(ICP-QqQ-MS),可以从凝胶中定量蛋白质结合的金属离子。我们确定了预计将与厌氧铵氧化和碳固定相关的大多数蛋白质复合物。ICP-MS / MS的数据表明,铁和锌存在于多种高分子量蛋白质复合物中(230-800 kDa)。在大小为500–650 kDa的蛋白质的凝胶切片中显着发现Mo,而仅使用去溶剂雾化器系统在350–500 kDa的蛋白质范围内发现Ni。检测到的蛋白质复合物及其金属含量与基因组注释一致。基于凝胶的金属蛋白质组学是表征金属蛋白的灵敏且可靠的方法,可用于表征生物系统中的许多多聚体金属蛋白复合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号