Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-10-12 , DOI: 10.1016/j.jmb.2020.10.003 Szymon W. Manka , Keith Brew
|
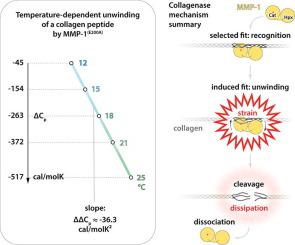
Local unwinding of the collagen triple helix is a necessary step for initiating the collagen degradation cascade in extracellular matrices. A few matrix metalloproteinases (MMPs) are known to support this key process, but its energetic aspects remain unknown. Here, we captured the thermodynamics of the triple helix unwinding by monitoring interactions between a collagen peptide and MMP-1(E200A) – an active-site mutant of an archetypal vertebrate collagenase – at increasing temperatures, using isothermal titration calorimetry (ITC). Coupled binding and unwinding manifests as a curved relationship between the total enthalpy change and temperature of the reaction, producing increasingly negative heat capacity change (ΔΔCp ≈ −36.3 kcal/molK2). A specially designed solid-phase binding and cleavage assay (SPBCA) reported strain in the catalytically relevant unwound state, suggesting that this state is distinct from the horizon of sampled conformations of the collagenase-susceptible site. MMP-1 appears to blend selected fit with induced fit mechanisms to catalyse collagen unwinding prior to cleavage of individual collagen chains.
中文翻译:

基质金属蛋白酶1对胶原蛋白的结合和解链的热力学和机械学见解
胶原三螺旋的局部展开是在细胞外基质中启动胶原降解级联反应的必要步骤。已知有几种基质金属蛋白酶(MMP)支持此关键过程,但其能量方面仍然未知。在这里,我们通过使用等温滴定量热(ITC)监测温度升高的温度下胶原肽与MMP-1(E200A)(原型脊椎动物胶原酶的活性位点突变)之间的相互作用来捕获三重螺旋展开的热力学。耦合结合和退绕清单作为反应的总焓变化和温度之间的弯曲的关系,产生越来越不利的热容量变化(ΔΔC p ≈-36.3千卡/ molK 2)。专门设计的固相结合和裂解测定法(SPBCA)报告了处于催化相关解链状态的菌株,表明该状态与胶原酶敏感性位点的采样构象不同。MMP-1似乎将选定的拟合与诱导的拟合机制混合在一起,以在切割单个胶原链之前催化胶原退绕。


























 京公网安备 11010802027423号
京公网安备 11010802027423号