Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-10-12 , DOI: 10.1016/j.jmb.2020.09.019 Victor E Cruz 1 , F Esra Demircioglu 1 , Thomas U Schwartz 1
|
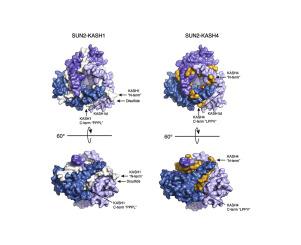
Linker of nucleoskeleton and cytoskeleton (LINC) complexes are molecular tethers that span the nuclear envelope (NE) and physically connect the nucleus to the cytoskeleton. They transmit mechanical force across the NE in processes such as nuclear anchorage, nuclear migration, and homologous chromosome pairing during meiosis. LINC complexes are composed of KASH proteins traversing the outer nuclear membrane, and SUN proteins crossing the inner nuclear membrane. Humans have several SUN- and KASH-containing proteins, yet what governs their proper engagement is poorly understood. To investigate this question, we solved high resolution crystal structures of human SUN2 in complex with the KASH-peptides of Nesprin3, Nesprin4, and KASH5. In comparison to the published structures of SUN2-KASH1/2 we observe alternative binding modes for these KASH peptides. While the core interactions between SUN and the C-terminal residues of the KASH peptide are similar in all five complexes, the extended KASH-peptide adopts at least two different conformations. The much-improved resolution allows for a more detailed analysis of other elements critical for KASH interaction, including the KASH-lid and the cation loop, and a possible self-locked state for unbound SUN. In summary, we observe distinct differences between the examined SUN-KASH complexes. These differences may have an important role in regulating the SUN-KASH network.
中文翻译:

不同 LINC 复合物的结构分析揭示了不同的结合模式
核骨架和细胞骨架 (LINC) 复合物的接头是跨越核膜 (NE) 并将细胞核物理连接到细胞骨架的分子系链。它们在减数分裂过程中的核锚定、核迁移和同源染色体配对等过程中通过 NE 传递机械力。LINC 复合物由穿过外核膜的 KASH 蛋白和穿过内核膜的 SUN 蛋白组成。人类有几种含有 SUN 和 KASH 的蛋白质,但人们对控制他们适当参与的因素知之甚少。为了研究这个问题,我们解决了与 Nesprin3、Nesprin4 和 KASH5 的 KASH 肽复合的人类 SUN2 的高分辨率晶体结构。与已发表的 SUN2-KASH1/2 结构相比,我们观察到这些 KASH 肽的替代结合模式。虽然 SUN 和 KASH 肽 C 端残基之间的核心相互作用在所有五种复合物中都相似,但扩展的 KASH 肽至少采用两种不同的构象。大大提高的分辨率允许更详细地分析对 KASH 相互作用至关重要的其他元素,包括 KASH 盖和阳离子环,以及未结合的 SUN 可能的自锁状态。总之,我们观察到所检查的 SUN-KASH 复合物之间的明显差异。这些差异可能对调节 SUN-KASH 网络具有重要作用。大大提高的分辨率允许更详细地分析对 KASH 相互作用至关重要的其他元素,包括 KASH 盖和阳离子环,以及未结合的 SUN 可能的自锁状态。总之,我们观察到所检查的 SUN-KASH 复合物之间的明显差异。这些差异可能对调节 SUN-KASH 网络具有重要作用。大大提高的分辨率允许更详细地分析对 KASH 相互作用至关重要的其他元素,包括 KASH 盖和阳离子环,以及未结合的 SUN 可能的自锁状态。总之,我们观察到所检查的 SUN-KASH 复合物之间的明显差异。这些差异可能对调节 SUN-KASH 网络具有重要作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号