Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2020-10-12 , DOI: 10.1016/j.cej.2020.127255 Gang Wang , Peng Wang , Huiling Liu , Jing Wang , Xiaohu Dai , Yanjun Xin
|
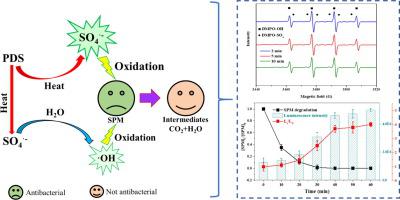
In recent years, antibiotic residues are frequently detected worldwide that has posed a serious threat to drinking water and increased the risk of bacterial resistance. Sulfate radical (SO4•−)-based advanced oxidation has been regarded as an effective technology for refractory organic pollutants treatment. In this study, the degradation kinetics and mechanism of spiramycin (SPM) under thermally activated peroxydisulfate (PDS) oxidation process in aqueous solution were investigated for the first time. The results indicated that the degradation rate of SPM could be expressed as the kinetic rate equation -d[SPM]/dt=(2.96×10-2 mM0 min-1)[SPM]0[SPM]1 within limited experimental conditions utilized here (i.e., 50 °C, pH 7, SPM 0.01-0.05 mM, and K2S2O8 1.0-2.72 mM). The apparent activation energy of 83.27 kJ·mol-1 was calculated by Arrhenius equation. The SPM degradation rate decreased with the increase of pH value. The SO4•− and hydroxyl radical (•OH) were proved to be the dominant reactive species, but the contribution of SO4•− on the SPM oxidation gradually decreased with the increase of pH value. The presence of humic acid (HA) and inorganic anions negatively affected the SPM degradation. To investigate the possible reaction pathways of SPM under thermally activated PDS system, HPLC/ESI-QqQMS was employed to identify the intermediate products. In addition, the acute toxicity evaluated by Vibrio fischeri showed that the oxidation byproducts of SPM were not antibacterial. In summary, this study confirmed that the thermally activated PDS technology could be a promising, efficient, and environmental-friendly approach for removing SPM in aqueous solution.
中文翻译:

热活化过氧二硫酸盐降解螺旋霉素的动力学研究,氧化产物和急性毒性
近年来,在世界范围内经常检测到抗生素残留,这对饮用水构成了严重威胁,并增加了细菌耐药性的风险。基于硫酸根(SO 4 •−)的高级氧化技术已被视为处理难处理有机污染物的有效技术。本研究首次研究了螺旋霉素在热活化过氧二硫酸盐(PDS)氧化过程中的降解动力学及其机理。结果表明,SPM的降解速率可以表示为动力学速率方程-d [SPM] / d t =(2.96×10 -2 mM 0 min -1)[SPM] 0 [SPM] 1在此处使用的有限实验条件下(即50°C,pH 7,SPM 0.01-0.05 mM和K 2 S 2 O 8 1.0-2.72 mM)。通过Arrhenius方程计算出表观活化能为83.27 kJ·mol -1。SPM降解率随pH值的增加而降低。事实证明,SO 4 •-和羟基自由基(•OH)是主要的反应性物质,但SO 4 •-的贡献随着pH值的升高,SPM的氧化逐渐降低。腐殖酸(HA)和无机阴离子的存在对SPM降解产生负面影响。为了研究热活化PDS系统下SPM的可能反应途径,采用HPLC / ESI-QqQMS鉴定中间产物。此外,费氏弧菌评估的急性毒性表明SPM的氧化副产物不是抗菌的。总而言之,这项研究证实了热活化PDS技术可能是一种去除水溶液中SPM的有前途,高效且环保的方法。

























 京公网安备 11010802027423号
京公网安备 11010802027423号