Catalysis Today ( IF 5.3 ) Pub Date : 2020-10-12 , DOI: 10.1016/j.cattod.2020.09.021 Xianghui Zhang , Neeru Chaudhary , Megan R. Hawkins , Cody B. Cockreham , Chen Yang , Junnan Shangguan , Alyssa J.R. Hensley , Ya-Huei (Cathy) Chin , Su Ha , Jean-Sabin McEwen , Di Wu
|
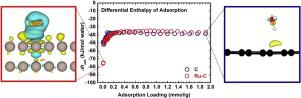
Fundamental knowledge on the energetics at the interface between a water layer and a metal catalyst is essential so as to understand the roles that water can play in the synthesis, activation and regeneration of noble metal-based catalysts. Here, we report the direct measurement of the enthalpy of water adsorption (Δhads) on activated carbon (C) and activated C-supported ruthenium (Ru) nanoparticles, which are promising catalyst as applied to the hydrogenation/hydrodeoxygenation (HDO) of oxygenates (phenolics, aldehydes, etc.). Specifically, the near-zero coverage enthalpy of water adsorption on a C-supported Ru catalyst is -75.3 ± 0.4 kJ/(mol water), suggesting favorable water–metal binding. This is much more exothermic than that on C, which has an enthalpy of adsorption of -50.3 ± 1.3 kJ/(mol water). Despite the favorable initial binding, the magnitudes of enthalpies of water condensation on C and Ru-C indicate that overall, their surfaces are both hydrophobic. Moreover, the experimentally-measured near-zero coverage water adsorption enthalpy at the Ru sites is in very good agreement with our density functional theory based calculations. At low coverages, we obtain a water binding energy of -61.7 kJ/(mol water), which increases to -78.1 kJ/(mol water) at saturation. Complementary results are also obtained from a thermal analysis, which employed a thermogravimetric analysis–mass spectrometry (TG-MS), a spectroscopic investigation using ex situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and a morphological evaluation with transmission electron microscopy (TEM). We point out that in carbon-supported metal catalysts, such as Ru-C, a strong hydration at near-zero coverage and relative weak water-surface interactions occurs upon saturation. Such heterogeneity is essential and crucial for catalytic hydrogenation/HDO reactions that involve balanced interactions among the water-rich reactant mixture and nonpolar organic products.
中文翻译:

确定碳载钌催化剂上的水合能:吸附量热法和密度泛函理论研究
为了了解水在贵金属基催化剂的合成,活化和再生中所起的作用,必须具备有关水层与金属催化剂之间界面的高能学的基础知识。在这里,我们报告水分的吸附焓直接测量(Δh的广告)在活性炭(C)和活性炭负载的钌(Ru)纳米颗粒上,它们有望应用于含氧化合物(酚类,醛类等)的加氢/加氢脱氧(HDO)。具体而言,在C负载的Ru催化剂上,水吸附的覆盖焓接近零,为-75.3±0.4 kJ /(mol水),表明水与金属的结合良好。这比在C上放热大得多,C的吸附焓为-50.3±1.3 kJ /(mol水)。尽管初始结合良好,但水在C和Ru-C上的冷凝焓值表明,总体而言,它们的表面均为疏水性的。此外,在Ru位置上通过实验测得的接近零覆盖率的水吸附焓与我们基于密度泛函理论的计算非常吻合。在低覆盖率的情况下,我们得到的水结合能为-61.7 kJ /(摩尔水),饱和时增加到-78.1 kJ /(摩尔水)。还可以通过热分析获得补充结果,该分析采用热重分析-质谱(TG-MS),即使用非原位漫反射红外傅里叶变换光谱(DRIFTS)和透射电子显微镜(TEM)的形态学评估。我们指出,在碳负载的金属催化剂(如Ru-C)中,饱和时会在接近零的覆盖率下发生强水合作用,而水-表面相互作用相对较弱。这种异质性对于催化加氢/ HDO反应至关重要,而催化加氢/ HDO反应涉及富水反应混合物与非极性有机产物之间的平衡相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号