当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation of Cu@Pd core@shell nanocatalysts with high activity for ethanol electro-oxidation in alkaline medium
Applied Surface Science ( IF 6.7 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2020.148119 J. Maya-Cornejo , J.A. Diaz-Real , Jose Luis Lopez-Miranda , Lorena Álvarez-Contreras , Rodrigo Esparza , Noé Arjona , Miriam Estévez
Applied Surface Science ( IF 6.7 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2020.148119 J. Maya-Cornejo , J.A. Diaz-Real , Jose Luis Lopez-Miranda , Lorena Álvarez-Contreras , Rodrigo Esparza , Noé Arjona , Miriam Estévez
|
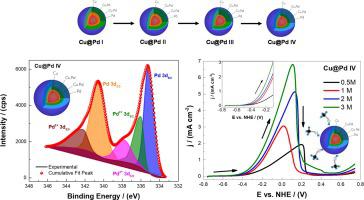
Abstract In this work, the formation of Cu@Pd core-shell nanoparticles was studied by optimizing the reduction of Cu2+ to Cu0 used as cores, and by increasing the amount of Pd precursor, labelling these materials as Cu@Pd I to IV, respectively. X-ray diffraction, UV-vis tests, and X-ray photoelectron spectroscopy (XPS) revealed the formation and progressive growth of Cu@Pd nanoparticles with the increase of the Pd content. High-resolution transmission electron microscopy (HR-TEM) and line-scan energy-dispersive spectroscopy (EDX line-scan) tests indicated that the Cu@Pd IV was the sample that displayed a better formation of a core@shell structure, having an average particle size of 5.9 nm. Electrochemical tests were performed using a synthesized Pd/C nanomaterial (15.7 nm) as reference electrocatalyst for the ethanol oxidation reaction (EOR), and the effect of the fuel and electrolyte concentrations were analyzed. The highest activity was obtained with 3 M ethanol + 2 M KOH, where Cu@Pd IV displayed an onset potential (Eonset) of −0.6 V vs. NHE and an oxidation peak potential (Ep) of 0.11 V. This Ep was 400 mV lower to that achieved by Pd/C at the same conditions. In addition, Cu@Pd IV presented the highest current density (6.78 mA cm−2, 1.6 times higher than Pd/C).
中文翻译:

碱性介质中乙醇电氧化高活性Cu@Pd核@壳纳米催化剂的形成
摘要 在这项工作中,通过优化将 Cu2+ 还原为用作核的 Cu0,并通过增加 Pd 前驱体的量,将这些材料分别标记为 Cu@Pd I 至 IV,研究了 Cu@Pd 核壳纳米粒子的形成。 . X 射线衍射、UV-vis 测试和 X 射线光电子能谱(XPS)揭示了随着 Pd 含量的增加,Cu@Pd 纳米颗粒的形成和逐渐生长。高分辨率透射电子显微镜 (HR-TEM) 和线扫描能量色散光谱 (EDX 线扫描) 测试表明,Cu@Pd IV 是显示出更好的核@壳结构形成的样品,具有平均粒径为 5.9 nm。使用合成的 Pd/C 纳米材料(15.7 nm)作为乙醇氧化反应(EOR)的参考电催化剂进行电化学测试,并分析了燃料和电解质浓度的影响。使用 3 M 乙醇 + 2 M KOH 获得最高活性,其中 Cu@Pd IV 显示出 -0.6 V 与 NHE 的起始电位(Eonset)和 0.11 V 的氧化峰电位(Ep)。该 Ep 为 400 mV低于相同条件下 Pd/C 所达到的水平。此外,Cu@Pd IV 呈现出最高的电流密度(6.78 mA cm-2,是 Pd/C 的 1.6 倍)。
更新日期:2021-02-01
中文翻译:

碱性介质中乙醇电氧化高活性Cu@Pd核@壳纳米催化剂的形成
摘要 在这项工作中,通过优化将 Cu2+ 还原为用作核的 Cu0,并通过增加 Pd 前驱体的量,将这些材料分别标记为 Cu@Pd I 至 IV,研究了 Cu@Pd 核壳纳米粒子的形成。 . X 射线衍射、UV-vis 测试和 X 射线光电子能谱(XPS)揭示了随着 Pd 含量的增加,Cu@Pd 纳米颗粒的形成和逐渐生长。高分辨率透射电子显微镜 (HR-TEM) 和线扫描能量色散光谱 (EDX 线扫描) 测试表明,Cu@Pd IV 是显示出更好的核@壳结构形成的样品,具有平均粒径为 5.9 nm。使用合成的 Pd/C 纳米材料(15.7 nm)作为乙醇氧化反应(EOR)的参考电催化剂进行电化学测试,并分析了燃料和电解质浓度的影响。使用 3 M 乙醇 + 2 M KOH 获得最高活性,其中 Cu@Pd IV 显示出 -0.6 V 与 NHE 的起始电位(Eonset)和 0.11 V 的氧化峰电位(Ep)。该 Ep 为 400 mV低于相同条件下 Pd/C 所达到的水平。此外,Cu@Pd IV 呈现出最高的电流密度(6.78 mA cm-2,是 Pd/C 的 1.6 倍)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号