当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NMR investigations on binding and dynamics of imidazolium-based ionic liquids with HEWL
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-10-09 , DOI: 10.1039/d0cp04584e R. Ravikanth Reddy 1, 2, 3, 4, 5 , Jithender G. Reddy 6, 7, 8, 9, 10 , B. V. N. Phani Kumar 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-10-09 , DOI: 10.1039/d0cp04584e R. Ravikanth Reddy 1, 2, 3, 4, 5 , Jithender G. Reddy 6, 7, 8, 9, 10 , B. V. N. Phani Kumar 1, 2, 3, 4, 5
Affiliation
|
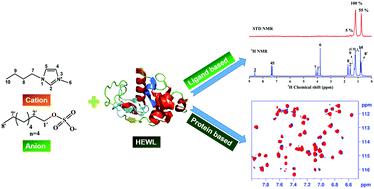
Molecular level insights on protein–ionic liquid (P–IL) interactions are beneficial for assessing protein stability, binding and dynamics. In the present work, interactions of ILs, namely, 1-butyl 3-methylimidazolium methyl sulfate (IL1), 1-butyl 3-methylimidazolium octyl sulfate (IL2) and 1-butyl 3-methylimidazolium chloride (IL3) with hen egg white lysozyme (HEWL) protein were investigated using solution-state nuclear magnetic resonance (NMR) spectroscopy. To ascertain the binding and dynamics from the perspective of both protein and IL, various ligand based NMR approaches such as selective and non-selective nuclear spin-relaxation (R1SEL and R1NS), saturation transfer difference (STD), difference of inversion recovery rate with and without target irradiation (DIRECTION), 35Cl line-shape and spin-relaxation, and protein back bone amide chemical shift perturbations (CSPs) from 1H–15N HSQC were utilized. Among the ILs investigated, IL2 experiences significant interaction relative to those of IL1 and IL3, as revealed by the combined R1SEL and R1NS analysis, which is further supported by STD NMR. CSP analyses of 1H–15N HSQC spectra of aqueous P–IL mixtures enabled to identify the potential binding sites of ILs with HEWL. Whereas, 15N longitudinal (R1) and transverse (R2) spin-relaxation rates and 15N{1H} heteronuclear nuclear Overhauser effect (hetNOE) data subjected to the model free analysis for IL2 yielded the rotational correlation times and order parameters of various residues of HEWL. Furthermore, the results could discern the nature of interactions between studied ILs and HEWL in terms of specific and non-specific interactions.
中文翻译:

咪唑类离子液体与HEWL结合和动力学的NMR研究
蛋白质-离子液体(P-IL)相互作用的分子水平见解有助于评估蛋白质的稳定性,结合力和动力学。在目前的工作中,IL,即1-丁基3-甲基咪唑甲基硫酸盐(IL1),1-丁基3-甲基咪唑辛基硫酸盐(IL2)和1-丁基3-甲基咪唑氯化物(IL3)与鸡蛋清溶菌酶的相互作用(HEWL)蛋白使用溶液态核磁共振(NMR)光谱进行了研究。为了从蛋白质和IL的角度确定结合和动力学,各种基于配体的NMR方法,例如选择性和非选择性核自旋松弛(R 1SEL和R 1NS),饱和转移差(STD),有和没有靶照射下的反转恢复率差异(DIRECTION),35 Cl线形和自旋弛豫以及蛋白背骨酰胺化学位移扰动(CSP)从1 H至15 N利用了HSQC。R 1SEL和R 1NS的组合分析表明,在所研究的IL中,IL2相对于IL1和IL3经历了显着的相互作用,这进一步得到了STD NMR的支持。对水性P–IL混合物的1 H– 15 N HSQC光谱进行CSP分析,可以鉴定IL与HEWL的潜在结合位点。而纵向15 N对模型IL2进行无模型分析的R 1)和横向(R 2)自旋弛豫速率以及15 N { 1 H}异核核Overhauser效应(hetNOE)数据得出了旋转相关时间和HEWL各种残基的顺序参数。此外,该结果可以根据特异性和非特异性相互作用来辨别研究的IL和HEWL之间相互作用的性质。
更新日期:2020-10-19
中文翻译:

咪唑类离子液体与HEWL结合和动力学的NMR研究
蛋白质-离子液体(P-IL)相互作用的分子水平见解有助于评估蛋白质的稳定性,结合力和动力学。在目前的工作中,IL,即1-丁基3-甲基咪唑甲基硫酸盐(IL1),1-丁基3-甲基咪唑辛基硫酸盐(IL2)和1-丁基3-甲基咪唑氯化物(IL3)与鸡蛋清溶菌酶的相互作用(HEWL)蛋白使用溶液态核磁共振(NMR)光谱进行了研究。为了从蛋白质和IL的角度确定结合和动力学,各种基于配体的NMR方法,例如选择性和非选择性核自旋松弛(R 1SEL和R 1NS),饱和转移差(STD),有和没有靶照射下的反转恢复率差异(DIRECTION),35 Cl线形和自旋弛豫以及蛋白背骨酰胺化学位移扰动(CSP)从1 H至15 N利用了HSQC。R 1SEL和R 1NS的组合分析表明,在所研究的IL中,IL2相对于IL1和IL3经历了显着的相互作用,这进一步得到了STD NMR的支持。对水性P–IL混合物的1 H– 15 N HSQC光谱进行CSP分析,可以鉴定IL与HEWL的潜在结合位点。而纵向15 N对模型IL2进行无模型分析的R 1)和横向(R 2)自旋弛豫速率以及15 N { 1 H}异核核Overhauser效应(hetNOE)数据得出了旋转相关时间和HEWL各种残基的顺序参数。此外,该结果可以根据特异性和非特异性相互作用来辨别研究的IL和HEWL之间相互作用的性质。


























 京公网安备 11010802027423号
京公网安备 11010802027423号