当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bioactivity improvement via display of the hydrophobic core of HYD1 in a cyclic β-hairpin-like scaffold, MTI-101
Peptide Science ( IF 2.4 ) Pub Date : 2020-10-09 , DOI: 10.1002/pep2.24199 Priyesh Jain 1, 2, 3 , David B Badger 1, 2 , Yi Liang 1 , Anthony W Gebhard 4 , Daniel Santiago 1 , Philip Murray 1 , Sridhar R Kaulagari 4, 5 , Ted J Gauthier 1, 6 , Rajesh Nair 4 , MohanRaja Kumar 1 , Wayne C Guida 1, 2 , Lori A Hazlehurst 3, 4, 5 , Mark L McLaughlin 1, 4, 5, 7
Peptide Science ( IF 2.4 ) Pub Date : 2020-10-09 , DOI: 10.1002/pep2.24199 Priyesh Jain 1, 2, 3 , David B Badger 1, 2 , Yi Liang 1 , Anthony W Gebhard 4 , Daniel Santiago 1 , Philip Murray 1 , Sridhar R Kaulagari 4, 5 , Ted J Gauthier 1, 6 , Rajesh Nair 4 , MohanRaja Kumar 1 , Wayne C Guida 1, 2 , Lori A Hazlehurst 3, 4, 5 , Mark L McLaughlin 1, 4, 5, 7
Affiliation
|
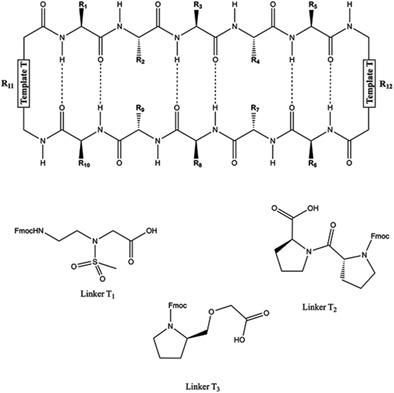
HYD1 is an all D-amino acid linear 10-mer peptide that was discovered by one-bead-one-compound screening. HYD1 has five hydrophobic amino acids flanked by polar amino acids. Alanine scanning studies showed that alternating hydrophobic amino acid residues and N- and C-terminal lysine side chains were contributors to the biological activity of the linear 10-mer analogs. This observation led us to hypothesize that display of the hydrophobic pentapeptide sequence of HYD1 in a cyclic beta-hairpin-like scaffold could lead to better bioavailability and biological activity. An amphipathic pentapeptide sequence was used to form an antiparallel strand and those strands were linked via dipeptide-like sequences selected to promote β-turns. Early cyclic analogs were more active but otherwise mimicked the biological activity of the linear HYD1 peptide. The cyclic peptidomimetics were synthesized using standard Fmoc solid phase synthesis to form linear peptides, followed by solution phase or on-resin cyclization. SAR studies were carried out with an aim to increase the potency of these drug candidates for the killing of multiple myeloma cells in vitro. The solution structures of 1, 5, and 10 were elucidated using NMR spectroscopy. 1H NMR and 2D TOCSY studies of these peptides revealed a downfield Hα proton chemical shift and 2D NOE spectral analysis consistent with a β-hairpin-like structure.
中文翻译:

通过在环状 β-发夹样支架 MTI-101 中展示 HYD1 的疏水核心提高生物活性
HYD1 是一种全 D 氨基酸线性 10 聚体肽,是通过单珠一化合物筛选发现的。HYD1 有五个疏水氨基酸,两侧是极性氨基酸。丙氨酸扫描研究表明,交替的疏水性氨基酸残基和 N 端和 C 端赖氨酸侧链是线性 10 聚体类似物的生物活性的贡献者。这一观察结果使我们假设在环状 β-发夹样支架中展示 HYD1 的疏水五肽序列可能会导致更好的生物利用度和生物活性。两亲五肽序列用于形成反平行链,这些链通过选择促进β-转角的二肽样序列连接。早期的环状类似物更具活性,但在其他方面模仿了线性 HYD1 肽的生物活性。使用标准 Fmoc 固相合成法合成环状肽模拟物以形成线性肽,然后进行溶液相或树脂上环化。进行 SAR 研究的目的是提高这些候选药物杀死多发性骨髓瘤细胞的效力体外。使用 NMR 光谱法阐明了1、5和10的溶液结构。这些肽的1 H NMR 和 2D TOCSY 研究揭示了与 β-发夹样结构一致的低场 H α质子化学位移和 2D NOE 光谱分析。
更新日期:2020-10-09
中文翻译:

通过在环状 β-发夹样支架 MTI-101 中展示 HYD1 的疏水核心提高生物活性
HYD1 是一种全 D 氨基酸线性 10 聚体肽,是通过单珠一化合物筛选发现的。HYD1 有五个疏水氨基酸,两侧是极性氨基酸。丙氨酸扫描研究表明,交替的疏水性氨基酸残基和 N 端和 C 端赖氨酸侧链是线性 10 聚体类似物的生物活性的贡献者。这一观察结果使我们假设在环状 β-发夹样支架中展示 HYD1 的疏水五肽序列可能会导致更好的生物利用度和生物活性。两亲五肽序列用于形成反平行链,这些链通过选择促进β-转角的二肽样序列连接。早期的环状类似物更具活性,但在其他方面模仿了线性 HYD1 肽的生物活性。使用标准 Fmoc 固相合成法合成环状肽模拟物以形成线性肽,然后进行溶液相或树脂上环化。进行 SAR 研究的目的是提高这些候选药物杀死多发性骨髓瘤细胞的效力体外。使用 NMR 光谱法阐明了1、5和10的溶液结构。这些肽的1 H NMR 和 2D TOCSY 研究揭示了与 β-发夹样结构一致的低场 H α质子化学位移和 2D NOE 光谱分析。



























 京公网安备 11010802027423号
京公网安备 11010802027423号