当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cyclization of Vinylketene Dithioacetals: A Synthetic Strategy for Substituted Thiophenes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-10-09 , DOI: 10.1002/adsc.202001001 Ling Pan 1 , Baihui Zheng 1 , Xiaohui Yang 1 , Liping Deng 1 , Yifei Li 1 , Qun Liu 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-10-09 , DOI: 10.1002/adsc.202001001 Ling Pan 1 , Baihui Zheng 1 , Xiaohui Yang 1 , Liping Deng 1 , Yifei Li 1 , Qun Liu 1
Affiliation
|
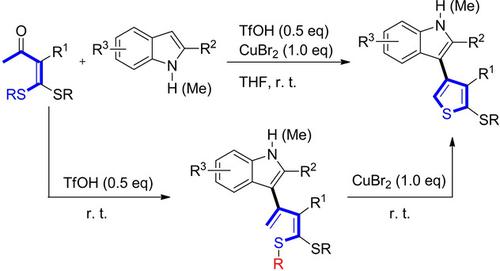
A synthetic strategy for the synthesis of substituted thiophenes is described by the one‐pot reaction of indoles with ketene dithioacetals under mild reaction conditions. Promoted by triflic acid (TfOH), the reaction of indoles with the easily available α‐acetyl ketene dithioacetals resulted in the formation of vinylketene dithioacetals via condensation instead of the well known nucleophilic addition‐alkylthio elimination process. In thepresence of CuBr2, vinylketene dithioacetals can cyclize into the corresponding substituted thiophenes. This transformation may benefit from the acidic reaction conditions and the steric effects of the 2‐substituted indoles.
中文翻译:

乙烯基乙烯酮二硫缩醛的环化:取代噻吩的合成策略
在温和的反应条件下,吲哚与乙烯酮二硫缩醛的单锅反应描述了合成取代噻吩的合成策略。在三氟甲磺酸(TfOH)的促进下,吲哚与易得的α-乙酰基乙烯酮二硫缩醛的反应导致乙烯基酮二硫缩醛通过缩合形成,而不是众所周知的亲核加成-烷硫基消除过程。在CuBr 2存在下,乙烯基乙烯酮二硫缩醛可以环化成相应的取代噻吩。这种转化可能得益于酸性反应条件和2-取代吲哚的空间位阻。
更新日期:2020-10-09
中文翻译:

乙烯基乙烯酮二硫缩醛的环化:取代噻吩的合成策略
在温和的反应条件下,吲哚与乙烯酮二硫缩醛的单锅反应描述了合成取代噻吩的合成策略。在三氟甲磺酸(TfOH)的促进下,吲哚与易得的α-乙酰基乙烯酮二硫缩醛的反应导致乙烯基酮二硫缩醛通过缩合形成,而不是众所周知的亲核加成-烷硫基消除过程。在CuBr 2存在下,乙烯基乙烯酮二硫缩醛可以环化成相应的取代噻吩。这种转化可能得益于酸性反应条件和2-取代吲哚的空间位阻。



























 京公网安备 11010802027423号
京公网安备 11010802027423号