当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Whole‐exome sequencing of T‐B+ severe combined immunodeficiency in Egyptian infants, JAK3 predominance and novel variants
Clinical & Experimental Immunology ( IF 4.6 ) Pub Date : 2020-10-10 , DOI: 10.1111/cei.13536 R El Hawary 1 , S Meshaal 1 , A A Mauracher 2 , L Opitz 3 , D Abd Elaziz 4 , S Lotfy 4 , A Eldash 1 , J Boutros 4 , N Galal 4 , J Pachlopnik Schmid 2 , A Elmarsafy 4
Clinical & Experimental Immunology ( IF 4.6 ) Pub Date : 2020-10-10 , DOI: 10.1111/cei.13536 R El Hawary 1 , S Meshaal 1 , A A Mauracher 2 , L Opitz 3 , D Abd Elaziz 4 , S Lotfy 4 , A Eldash 1 , J Boutros 4 , N Galal 4 , J Pachlopnik Schmid 2 , A Elmarsafy 4
Affiliation
|
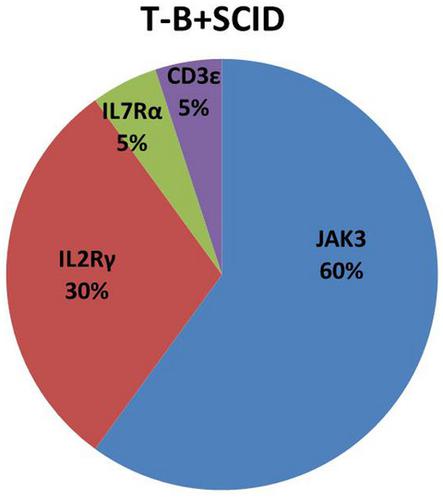
Severe combined immunodeficiency (SCID) is fatal if not treated with immune reconstitution. In Egypt, T‐B+ SCID accounts for 38·5% of SCID diagnoses. An accurate genetic diagnosis is essential for choosing appropriate treatment modalities and for offering genetic counseling to the patient’s family. The objectives of this study were to describe the clinical, immunological and molecular characteristics of a cohort of twenty Egyptian patients with T‐B+ SCID. The initial diagnosis (based on clinical features and flow cytometry) was followed by molecular investigation (whole‐exome sequencing). All patients had the classic clinical picture for SCID, including failure to thrive (n = 20), oral candidiasis (n = 17), persistent diarrhea (n = 14), pneumonia (n = 13), napkin dermatitis (n = 10), skin rash (n = 7), otitis media (n = 3) and meningitis (n = 2). The onset of manifestations was at the age of 2·4 ± 1·6 months and diagnosis at the age of 6·7 ± ·5 months, giving a diagnostic delay of 4·3 months. JAK3 gene variants were most frequent (n = 12) with three novel variants identified, followed by IL2Rγ variants (n = 6) with two novel variants. IL7Rα and CD3ε variants were found once, with a novel variant each. T‐B+NK− SCID accounted for approximately 90% of the Egyptian patients with T‐B+SCID. Of these T‐B+NK− SCID cases, 60% were autosomal recessive syndromes caused by JAK3 mutations and 30% were X‐linked syndromes. It might be useful to sequence the JAK3 gene (i.e. targeted Sanger sequencing) in all T‐B+ SCID patients, especially after X‐linked SCID has been ruled out. Hence, no more than 10% of T‐B+ SCID patients might require next‐generation for a molecular diagnosis.
中文翻译:

埃及婴儿 T-B+ 严重联合免疫缺陷的全外显子组测序、JAK3 优势和新变体
如果不进行免疫重建治疗,严重的联合免疫缺陷 (SCID) 是致命的。在埃及,T ‐ B + SCID 占 SCID 诊断的 38·5%。准确的遗传诊断对于选择适当的治疗方式和为患者家属提供遗传咨询至关重要。本研究的目的是描述一组 20 名埃及 T - B + SCID患者的临床、免疫学和分子特征。初步诊断(基于临床特征和流式细胞术)之后是分子研究(全外显子组测序)。所有患者都有 SCID 的典型临床表现,包括发育迟缓(n = 20)、口腔念珠菌病(n = 17)、持续性腹泻 ( n = 14)、肺炎 ( n = 13)、餐巾皮炎 ( n = 10)、皮疹 ( n = 7)、中耳炎 ( n = 3) 和脑膜炎 ( n = 2) . 2·4±1·6个月发病,6·7±·5个月诊断,诊断延迟4·3个月。JAK 3 基因变体最常见 ( n = 12),鉴定出三个新变体,其次是IL2R γ 变体 ( n = 6),有两个新变体。IL7Rα和CD3ε变体被发现一次,每个都有一个新的变体。吨- B + NK - SCID 约占埃及 T - B + SCID 患者的 90%。在这些 T - B + NK - SCID 病例中,60% 是由JAK3突变引起的常染色体隐性遗传综合征,30% 是 X 连锁综合征。在所有 T ‐ B + SCID 患者中对JAK3基因进行测序(即靶向 Sanger 测序)可能有用,尤其是在 X 连锁 SCID 已被排除后。因此,不超过 10% 的 T - B + SCID 患者可能需要下一代进行分子诊断。
更新日期:2020-10-10
中文翻译:

埃及婴儿 T-B+ 严重联合免疫缺陷的全外显子组测序、JAK3 优势和新变体
如果不进行免疫重建治疗,严重的联合免疫缺陷 (SCID) 是致命的。在埃及,T ‐ B + SCID 占 SCID 诊断的 38·5%。准确的遗传诊断对于选择适当的治疗方式和为患者家属提供遗传咨询至关重要。本研究的目的是描述一组 20 名埃及 T - B + SCID患者的临床、免疫学和分子特征。初步诊断(基于临床特征和流式细胞术)之后是分子研究(全外显子组测序)。所有患者都有 SCID 的典型临床表现,包括发育迟缓(n = 20)、口腔念珠菌病(n = 17)、持续性腹泻 ( n = 14)、肺炎 ( n = 13)、餐巾皮炎 ( n = 10)、皮疹 ( n = 7)、中耳炎 ( n = 3) 和脑膜炎 ( n = 2) . 2·4±1·6个月发病,6·7±·5个月诊断,诊断延迟4·3个月。JAK 3 基因变体最常见 ( n = 12),鉴定出三个新变体,其次是IL2R γ 变体 ( n = 6),有两个新变体。IL7Rα和CD3ε变体被发现一次,每个都有一个新的变体。吨- B + NK - SCID 约占埃及 T - B + SCID 患者的 90%。在这些 T - B + NK - SCID 病例中,60% 是由JAK3突变引起的常染色体隐性遗传综合征,30% 是 X 连锁综合征。在所有 T ‐ B + SCID 患者中对JAK3基因进行测序(即靶向 Sanger 测序)可能有用,尤其是在 X 连锁 SCID 已被排除后。因此,不超过 10% 的 T - B + SCID 患者可能需要下一代进行分子诊断。


























 京公网安备 11010802027423号
京公网安备 11010802027423号