当前位置:
X-MOL 学术
›
Thermochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic study of the thermo-oxidative decomposition of metformin by isoconversional and theoretical methods
Thermochimica Acta ( IF 3.5 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.tca.2020.178797 Ismail Badran , Abdallah D. Manasrah , Azfar Hassan , Nashaat N. Nassar
Thermochimica Acta ( IF 3.5 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.tca.2020.178797 Ismail Badran , Abdallah D. Manasrah , Azfar Hassan , Nashaat N. Nassar
|
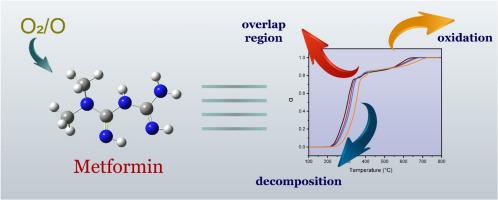
Abstract The drug metformin is the most prescribed drug to treat type II diabetes and has been recently reported to have anticancer activities. Because of its wide use, its potential risk on the environment is extremely concerning. In this study, the mechanism and the thermodynamics of the thermo-oxidative decomposition of the metformin were investigated as part of a new solution for the pharmaceutical contamination of water bodies. Thermogravimetry and mass spectrometry were used to demonstrate the metformin thermo-oxidative decomposition under air in the temperature range 25 to 800 °C. The isoconversional methods of Kissinger-Akahira Sunose (KAS) and Friedman (FR) were implemented to deduce the trends of effective activation energies. As expected, the effective activation energy (Eα) of the reaction was dependent on the reaction temperature, suggesting multi-step reactions. The Eα ranged from 100 to 145 kJ/mol and 200 to 300 kJ/mol for the KAS and FR methods, respectively. The kinetic triplet, Aα, ΔS‡, and ΔG‡ were also determined by finding the appropriate reaction model. Theoretical calculations were implemented to propose a full reaction mechanism. The oxidation of metformin was investigated with both molecular O2(t) and atomic O(t) oxygen. The experimental results were then explained under the light of the computational data to explain the variation of Eα with temperature, and the competition between the O2(t)/O(t) species.
中文翻译:

二甲双胍热氧化分解的等转化和理论方法动力学研究
摘要 二甲双胍是治疗 II 型糖尿病最常用的药物,最近有报道称其具有抗癌活性。由于其广泛使用,其对环境的潜在风险非常令人担忧。在这项研究中,研究了二甲双胍热氧化分解的机制和热力学,作为解决水体药物污染的新解决方案的一部分。使用热重法和质谱法来证明二甲双胍在 25 至 800 °C 温度范围内在空气中的热氧化分解。实施了 Kissinger-Akahira Sunose (KAS) 和 Friedman (FR) 的等转化方法来推断有效活化能的趋势。正如预期的那样,反应的有效活化能 (Eα) 取决于反应温度,建议多步反应。对于 KAS 和 FR 方法,Eα 的范围分别为 100 至 145 kJ/mol 和 200 至 300 kJ/mol。动力学三重态 Aα、ΔS‡ 和 ΔG‡ 也通过寻找合适的反应模型来确定。实施理论计算以提出完整的反应机制。用分子 O2(t) 和原子 O(t) 氧研究二甲双胍的氧化。然后根据计算数据解释实验结果,以解释 Eα 随温度的变化以及 O2(t)/O(t) 物种之间的竞争。实施理论计算以提出完整的反应机制。用分子 O2(t) 和原子 O(t) 氧研究二甲双胍的氧化。然后根据计算数据解释实验结果,以解释 Eα 随温度的变化以及 O2(t)/O(t) 物种之间的竞争。实施理论计算以提出完整的反应机制。用分子 O2(t) 和原子 O(t) 氧研究二甲双胍的氧化。然后根据计算数据解释实验结果,以解释 Eα 随温度的变化以及 O2(t)/O(t) 物种之间的竞争。
更新日期:2020-12-01
中文翻译:

二甲双胍热氧化分解的等转化和理论方法动力学研究
摘要 二甲双胍是治疗 II 型糖尿病最常用的药物,最近有报道称其具有抗癌活性。由于其广泛使用,其对环境的潜在风险非常令人担忧。在这项研究中,研究了二甲双胍热氧化分解的机制和热力学,作为解决水体药物污染的新解决方案的一部分。使用热重法和质谱法来证明二甲双胍在 25 至 800 °C 温度范围内在空气中的热氧化分解。实施了 Kissinger-Akahira Sunose (KAS) 和 Friedman (FR) 的等转化方法来推断有效活化能的趋势。正如预期的那样,反应的有效活化能 (Eα) 取决于反应温度,建议多步反应。对于 KAS 和 FR 方法,Eα 的范围分别为 100 至 145 kJ/mol 和 200 至 300 kJ/mol。动力学三重态 Aα、ΔS‡ 和 ΔG‡ 也通过寻找合适的反应模型来确定。实施理论计算以提出完整的反应机制。用分子 O2(t) 和原子 O(t) 氧研究二甲双胍的氧化。然后根据计算数据解释实验结果,以解释 Eα 随温度的变化以及 O2(t)/O(t) 物种之间的竞争。实施理论计算以提出完整的反应机制。用分子 O2(t) 和原子 O(t) 氧研究二甲双胍的氧化。然后根据计算数据解释实验结果,以解释 Eα 随温度的变化以及 O2(t)/O(t) 物种之间的竞争。实施理论计算以提出完整的反应机制。用分子 O2(t) 和原子 O(t) 氧研究二甲双胍的氧化。然后根据计算数据解释实验结果,以解释 Eα 随温度的变化以及 O2(t)/O(t) 物种之间的竞争。


























 京公网安备 11010802027423号
京公网安备 11010802027423号