Physica E: Low-dimensional Systems and Nanostructures ( IF 3.3 ) Pub Date : 2020-10-09 , DOI: 10.1016/j.physe.2020.114473 Sadegh Kaviani , Siyamak Shahab , Masoome Sheikhi
|
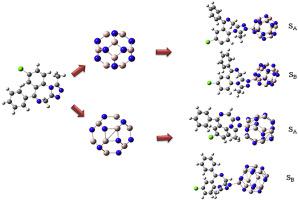
In this study, density functional theory (DFT) calculations were used to investigate the interaction of the B12N12 and Al12N12 nano-cages with alprazolam (ALP) in the gas phase and water at the B3LYP/6-31G(d,p) level of the theory. According to the obtained results, B12N12 nano-cage can be able to form a more stable complex with ALP in comparison to Al12N12. The stability of ALP/B12N12 and ALP/Al12N12 complexes was increased in the water as a simulation of the body condition. Natural bond orbital (NBO) analysis revealed an effective charge transfer from the nitrogen atoms of ALP to the boron atoms of nano-cages. Quantum theory of atoms in molecules (QTAIM) analysis indicated that the non-covalent interactions between Al12N12 and ALP are the main driving force in the complex formation, whereas ALP/B12N12 interaction is mainly electrostatic and partial covalent in nature. Moreover, there is an intra-molecular hydrogen bond between nitrogen atom of the nano-cages and hydrogen atom of ALP. Finally frontier molecular orbital (FMO) analysis was performed to get a better insight of the reactivity and stability of ALP/nano-cage complexes in the gas phase and water. Based on FMO analysis, B12N12 nano-cage is a better biosensor for the detection of ALP drug in comparison to Al12N12 due to the higher changes in its band gap.
中文翻译:

DFT研究:阿普唑仑药物在B 12 N 12和Al 12 N 12纳米笼上的生物吸附
在这项研究中,使用密度泛函理论(DFT)计算来研究B 12 N 12和Al 12 N 12纳米笼与阿普唑仑(ALP)在气相和水中在B3LYP / 6-31G下的相互作用( d,p)理论水平。根据获得的结果,与Al 12 N 12相比,B 12 N 12纳米笼能够与ALP形成更稳定的配合物。ALP / B 12 N 12和ALP / Al 12 N 12的稳定性人体中的模拟物增加了水中的复合物。自然键轨道(NBO)分析显示了从ALP的氮原子到纳米笼子的硼原子的有效电荷转移。分子中的原子量子理论(QTAIM)分析表明,Al 12 N 12与ALP之间的非共价相互作用是形成复合物的主要驱动力,而ALP / B 12 N 12相互作用主要是静电和部分共价。此外,在纳米笼的氮原子和ALP的氢原子之间存在分子内氢键。最后,进行了前沿分子轨道(FMO)分析,以更好地了解ALP /纳米笼络合物在气相和水中的反应性和稳定性。基于FMO分析,与Al 12 N 12相比,B 12 N 12纳米笼由于其带隙变化较大,是用于检测ALP药物的更好的生物传感器。


























 京公网安备 11010802027423号
京公网安备 11010802027423号