当前位置:
X-MOL 学术
›
Mater. Today Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
First-row transition-metal-doped graphyne for ultrahigh-performance CO2 capture and separation over N2/CH4/H2
Materials Today Physics ( IF 11.5 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.mtphys.2020.100301 S. Zhou , M. Wang , S. Wei , S. Cao , Z. Wang , S. Liu , D. Sun , X. Lu
Materials Today Physics ( IF 11.5 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.mtphys.2020.100301 S. Zhou , M. Wang , S. Wei , S. Cao , Z. Wang , S. Liu , D. Sun , X. Lu
|
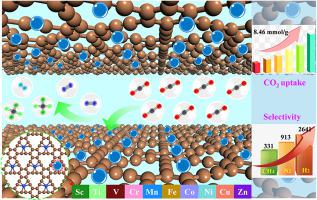
Abstract Exploring new materials with high-performance CO2 capture and separation is critical for the development of CO2 capture and utilization strategy to alleviate the excessive CO2 emissions in the atmosphere. Herein, first-row transition-metal-doped graphynes were screened according to their stability and surface flatness, and Cu-GY, Co-GY, Fe-GY, and Mn-GY were systematically evaluated. Structural analyses showed that the four TM-GYs had high cohesive energies ranging from 7.07 to 7.35 eV/atom, moderate formation energies ranging from −6.83 to −3.28 eV, large diffusion barriers of the TM atoms ranging from 2.25 to 3.48 eV, which guaranteed their structural stabilities for CO2 capture and separation. Electronic structure analyses confirmed the evident orbital overlap, large charge transfer, and strong covalent bonding characters between the TMs and direct-connected C atoms, thus constructing a favorable gas adsorption environment. Cu-GY exhibited ultrahigh CO2 adsorption capacity of 8.46 mmol/g at 298 K and 1.0 bar, which was larger than ever reported results in slit pores and comparable to the highest adsorption capacity of 8.60 mmol/g in Mg-MOF-74 under the same conditions. At 298 K and 1.0 bar, the selectivities of CO2 over N2/CH4/H2 in Cu-GY reached up to 913, 331, and 2641, respectively. Interaction analyses proved that the CO2 interaction with frameworks via both Coulomb and van der Waals was greater than those of the other gases. Strong affinity of CO2 with Cu-GY relative to other TM-GYs and large isosteric heat differences between CO2 and other gases rendered Cu-GY to possess the ultrahigh CO2 adsorption capacity and remarkable selectivity over N2/CH4/H2. Gas distribution analyses exhibited the wide CO2 distribution composed of multilayer adsorption peaks around the TMs and adjacent C atoms, elucidating the significant effect of the TMs and TM-connected C atoms on CO2 adsorption. Results of this work highlighted TM-GYs as ultrahigh-performance adsorbents for CO2 capture and separation over N2/CH4/H2.
中文翻译:

第一排过渡金属掺杂石墨炔用于在 N2/CH4/H2 上进行超高性能 CO2 捕获和分离
摘要 探索具有高性能 CO2 捕获和分离的新材料对于制定 CO2 捕获和利用策略以缓解大气中过量的 CO2 排放至关重要。在此,第一排过渡金属掺杂石墨烯根据其稳定性和表面平整度进行筛选,并对 Cu-GY、Co-GY、Fe-GY 和 Mn-GY 进行了系统评估。结构分析表明,四个 TM-GY 具有 7.07 至 7.35 eV/原子的高内聚能,-6.83 至 -3.28 eV 的中等形成能,2.25 至 3.48 eV 的大扩散势垒,这保证了它们对 CO2 捕获和分离的结构稳定性。电子结构分析证实了明显的轨道重叠、大的电荷转移、TMs与直接连接的C原子之间具有强共价键合特性,从而构建了良好的气体吸附环境。Cu-GY 在 298 K 和 1.0 bar 下表现出 8.46 mmol/g 的超高 CO2 吸附容量,这比以往报道的狭缝孔的结果更大,可与 Mg-MOF-74 在 Mg-MOF-74 下的最高吸附容量 8.60 mmol/g 相媲美相同的条件。在 298 K 和 1.0 bar 下,Cu-GY 中 CO2 对 N2/CH4/H2 的选择性分别达到 913、331 和 2641。相互作用分析证明,CO2 通过库仑和范德瓦尔斯与骨架的相互作用大于其他气体。相对于其他 TM-GY,CO2 与 Cu-GY 的强亲和力以及 CO2 与其他气体之间较大的等量热差使 Cu-GY 具有超高的 CO2 吸附能力和对 N2/CH4/H2 的显着选择性。气体分布分析显示出由围绕 TM 和相邻 C 原子的多层吸附峰组成的宽 CO2 分布,阐明了 TM 和 TM 连接的 C 原子对 CO2 吸附的显着影响。这项工作的结果突出了 TM-GY 作为超高性能吸附剂,用于通过 N2/CH4/H2 捕获和分离 CO2。
更新日期:2021-01-01
中文翻译:

第一排过渡金属掺杂石墨炔用于在 N2/CH4/H2 上进行超高性能 CO2 捕获和分离
摘要 探索具有高性能 CO2 捕获和分离的新材料对于制定 CO2 捕获和利用策略以缓解大气中过量的 CO2 排放至关重要。在此,第一排过渡金属掺杂石墨烯根据其稳定性和表面平整度进行筛选,并对 Cu-GY、Co-GY、Fe-GY 和 Mn-GY 进行了系统评估。结构分析表明,四个 TM-GY 具有 7.07 至 7.35 eV/原子的高内聚能,-6.83 至 -3.28 eV 的中等形成能,2.25 至 3.48 eV 的大扩散势垒,这保证了它们对 CO2 捕获和分离的结构稳定性。电子结构分析证实了明显的轨道重叠、大的电荷转移、TMs与直接连接的C原子之间具有强共价键合特性,从而构建了良好的气体吸附环境。Cu-GY 在 298 K 和 1.0 bar 下表现出 8.46 mmol/g 的超高 CO2 吸附容量,这比以往报道的狭缝孔的结果更大,可与 Mg-MOF-74 在 Mg-MOF-74 下的最高吸附容量 8.60 mmol/g 相媲美相同的条件。在 298 K 和 1.0 bar 下,Cu-GY 中 CO2 对 N2/CH4/H2 的选择性分别达到 913、331 和 2641。相互作用分析证明,CO2 通过库仑和范德瓦尔斯与骨架的相互作用大于其他气体。相对于其他 TM-GY,CO2 与 Cu-GY 的强亲和力以及 CO2 与其他气体之间较大的等量热差使 Cu-GY 具有超高的 CO2 吸附能力和对 N2/CH4/H2 的显着选择性。气体分布分析显示出由围绕 TM 和相邻 C 原子的多层吸附峰组成的宽 CO2 分布,阐明了 TM 和 TM 连接的 C 原子对 CO2 吸附的显着影响。这项工作的结果突出了 TM-GY 作为超高性能吸附剂,用于通过 N2/CH4/H2 捕获和分离 CO2。


























 京公网安备 11010802027423号
京公网安备 11010802027423号