当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect and Mechanism of 1-Hexyl-3-methylimidazolium-Based Ionic Liquids on the Isobaric Vapor–Liquid Equilibria of Methanol + Acetonitrile at 101.3 kPa
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-10-08 , DOI: 10.1021/acs.jced.0c00532 Jiujuan Zhu 1 , Hanhan Fan 1 , Bing Sun 1 , Hongbin Qi 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-10-08 , DOI: 10.1021/acs.jced.0c00532 Jiujuan Zhu 1 , Hanhan Fan 1 , Bing Sun 1 , Hongbin Qi 1
Affiliation
|
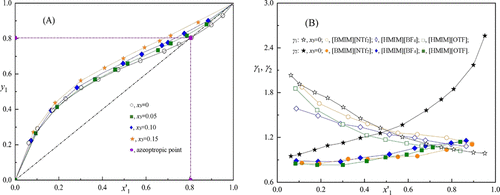
This work studied the separation effect of 1-hexyl-3-methylimidazolium-based ionic liquids (1-hexyl-3-methylimidazolium tetrafluoroborate [HMIM][BF4] and 1-hexyl-3-methylimidazolium trifluoromethanesulfonate [HMIM][OTF]) to separate a methanol and acetonitrile azeotropic system. The isobaric vapor–liquid equilibrium data obtained for the ternary systems {methanol + acetonitrile + ([HMIM][BF4] or [HMIM][OTF])} were measured at 101.3 kPa. Based on the nonrandom two-liquid model, the minimum mole fractions of [HMIM][BF4] and [HMIM][OTF] required to eliminate the azeotrope point of the methanol + acetonitrile mixtures were 0.047 and 0.042, respectively. Through the analysis of the σ-profiles and separation ability, [HMIM][OTF] was a suitable entrainer to separate the methanol–acetonitrile azeotrope.
中文翻译:

1-己基-3-甲基咪唑鎓离子液体对甲醇和乙腈在101.3 kPa的等压蒸气-液体平衡的影响及其机理
这项工作研究了基于1-己基-3-甲基咪唑鎓的离子液体(1-己基-3-甲基咪唑鎓四氟硼酸酯[HMIM] [BF 4 ]和1-己基-3-甲基咪唑鎓三氟甲烷磺酸酯[HMIM] [OTF])的分离效果。分离甲醇和乙腈共沸体系。在101.3 kPa下测量了三元体系{甲醇+乙腈+([HMIM] [BF 4 ]或[HMIM] [OTF])的等压气液平衡数据。基于非随机两液模型,[HMIM] [BF 4消除甲醇+乙腈混合物的共沸点所需的]和[HMIM] [OTF]分别为0.047和0.042。通过分析σ分布和分离能力,[HMIM] [OTF]是分离甲醇-乙腈共沸物的合适夹带剂。
更新日期:2020-11-12
中文翻译:

1-己基-3-甲基咪唑鎓离子液体对甲醇和乙腈在101.3 kPa的等压蒸气-液体平衡的影响及其机理
这项工作研究了基于1-己基-3-甲基咪唑鎓的离子液体(1-己基-3-甲基咪唑鎓四氟硼酸酯[HMIM] [BF 4 ]和1-己基-3-甲基咪唑鎓三氟甲烷磺酸酯[HMIM] [OTF])的分离效果。分离甲醇和乙腈共沸体系。在101.3 kPa下测量了三元体系{甲醇+乙腈+([HMIM] [BF 4 ]或[HMIM] [OTF])的等压气液平衡数据。基于非随机两液模型,[HMIM] [BF 4消除甲醇+乙腈混合物的共沸点所需的]和[HMIM] [OTF]分别为0.047和0.042。通过分析σ分布和分离能力,[HMIM] [OTF]是分离甲醇-乙腈共沸物的合适夹带剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号