Journal of Structural Biology ( IF 3 ) Pub Date : 2020-10-08 , DOI: 10.1016/j.jsb.2020.107636 Amin Mansoorifar 1 , Ramesh Subbiah 1 , Gabriela de Souza Balbinot 2 , Selvakumar Prakash Parthiban 1 , Luiz E Bertassoni 3
|
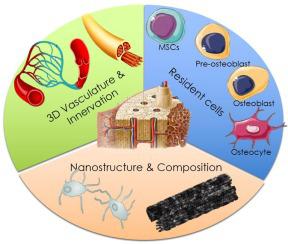
Bone mineralization is a highly specific and dynamic nanoscale process that has been studied extensively from a structural, chemical, and biological standpoint. Bone tissue, therefore, may be defined by the interplay of its intricately mineralized matrix and the cells that regulate its biological function. However, the far majority of engineered bone model systems and bone replacement materials have been unable to replicate this key characteristic of bone tissue; that is, the ability of cells to be gradually and rapidly embedded in a three-dimensional (3D) heavily calcified matrix material. Here we review the characteristics that define the bone matrix from a nanostructural perspective. We then revisit the benefits and challenges of existing model systems and engineered bone replacement materials, and discuss recent efforts to replicate the biological, cellular, mechanical, and materials characteristics of bone tissue on the nano- to microscale. We pay particular attention to a recently proposed method developed by our group, which seeks to replicate key aspects of the entrapment of bone cells within a mineralized matrix with precisions down to the level of individual nano-crystallites, inclusive of the bone vasculature, and osteogenic differentiation process. In summary, this paper discusses existing and emerging evidence pointing towards future developments bridging the gap between the fields of biomineralization, structural biology, stem cells, and tissue engineering, which we believe will hold the key to engineer truly functional bone-like tissue in the laboratory.
中文翻译:

将细胞嵌入纳米级、快速矿化的水凝胶:一种设计载有细胞的骨样组织的新范式
骨矿化是一种高度特异性和动态的纳米级过程,已从结构、化学和生物学的角度进行了广泛的研究。因此,骨组织可以通过其复杂的矿化基质和调节其生物功能的细胞的相互作用来定义。然而,绝大多数工程骨模型系统和骨替代材料都无法复制骨组织的这一关键特征;也就是说,细胞能够逐渐快速地嵌入三维 (3D) 高度钙化的基质材料中的能力。在这里,我们从纳米结构的角度回顾了定义骨基质的特征。然后我们重新审视现有模型系统和工程骨替代材料的好处和挑战,并讨论最近在纳米到微米尺度上复制骨组织的生物学、细胞、机械和材料特性的努力。我们特别关注我们小组最近提出的一种方法,该方法旨在复制矿化基质中骨细胞截留的关键方面,精确到单个纳米微晶的水平,包括骨血管系统和成骨分化过程。总而言之,本文讨论了现有和新出现的证据,这些证据指向未来的发展,弥合生物矿化、结构生物学、干细胞和组织工程领域之间的差距,我们相信这些领域将是设计真正功能性骨样组织的关键。实验室。纳米到微米尺度的骨组织和材料特性。我们特别关注我们小组最近提出的一种方法,该方法旨在复制矿化基质中骨细胞截留的关键方面,精确到单个纳米微晶的水平,包括骨血管系统和成骨分化过程。总而言之,本文讨论了现有和新出现的证据,这些证据指向未来的发展,弥合生物矿化、结构生物学、干细胞和组织工程领域之间的差距,我们相信这些领域将是设计真正功能性骨样组织的关键。实验室。纳米到微米尺度的骨组织和材料特性。我们特别关注我们小组最近提出的一种方法,该方法旨在复制矿化基质中骨细胞截留的关键方面,精确到单个纳米微晶的水平,包括骨血管系统和成骨分化过程。总而言之,本文讨论了现有和新出现的证据,这些证据指向未来的发展,弥合生物矿化、结构生物学、干细胞和组织工程领域之间的差距,我们相信这些领域将是设计真正功能性骨样组织的关键。实验室。它试图复制矿化基质中骨细胞截留的关键方面,精确到单个纳米微晶的水平,包括骨脉管系统和成骨分化过程。总而言之,本文讨论了现有和新出现的证据,这些证据指向未来的发展,弥合生物矿化、结构生物学、干细胞和组织工程领域之间的差距,我们相信这些领域将是设计真正功能性骨样组织的关键。实验室。它试图复制矿化基质中骨细胞截留的关键方面,精确到单个纳米微晶的水平,包括骨血管系统和成骨分化过程。总而言之,本文讨论了现有和新出现的证据,这些证据指向未来的发展,弥合生物矿化、结构生物学、干细胞和组织工程领域之间的差距,我们相信这些领域将是设计真正功能性骨样组织的关键。实验室。


























 京公网安备 11010802027423号
京公网安备 11010802027423号