当前位置:
X-MOL 学术
›
Diam. Relat. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pristine Detonation Nanodiamonds as Regenerable Adsorbents for Metal Cations
Diamond and Related Materials ( IF 4.1 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.diamond.2020.108121 Dmitry S. Volkov , Petr K. Krivoshein , Ivan V. Mikheev , Mikhail A. Proskurnin
Diamond and Related Materials ( IF 4.1 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.diamond.2020.108121 Dmitry S. Volkov , Petr K. Krivoshein , Ivan V. Mikheev , Mikhail A. Proskurnin
|
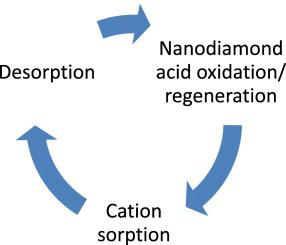
Abstract The usability of pristine (not functionalized by grafting) detonation nanodiamonds as reversible and repeatedly regenerated adsorbents for metal cations was shown by examples of sorption of cadmium, zinc, nickel, and copper from aqueous solutions. Various pristine nanodiamond surface treatments are discussed. The procedure of oxidative sintering with nitric acid at 250 °C provided a surface with an increased number of carboxyl groups with a sorption capacity above 100 μmol/g for all test cations. Sorption isotherms for test metals (copper, nickel, zinc, and cadmium) can be divided into two distinct parts: a lower part for concentrations of up to 10–15 mmol/L belong to the Langmuir L5 type by Giles and absorption free energies are 8–12 kJ/mol according to the Dubinin–Radushkevich model, evidencing a primarily chemisorption mechanism of the studied cations on micropores in ND surface. The parts of curves above 10–15 mmol/L are described with linear equations, free energies of absorption are 4–6 kJ/mol by Dubinin–Radushkevich that are assigned to physisorption in mesopores. A procedure for the surface regeneration, which is based a similar sintering treatment with nitric acid, was developed. It provided sorption parameters not degraded compared to the starting material; and the regeneration process is repeatable at least for 10 cycles.
中文翻译:

原始爆炸纳米金刚石作为金属阳离子的可再生吸附剂
摘要 通过从水溶液中吸附镉、锌、镍和铜的例子展示了原始(未通过接枝功能化)爆炸纳米金刚石作为金属阳离子的可逆和重复再生吸附剂的可用性。讨论了各种原始纳米金刚石表面处理。在 250 °C 下用硝酸氧化烧结的过程提供了一个具有更多羧基的表面,对所有测试阳离子的吸附容量都超过 100 μmol/g。测试金属(铜、镍、锌和镉)的吸附等温线可分为两个不同的部分:浓度高达 10-15 mmol/L 的较低部分属于 Giles 的 Langmuir L5 型,吸收自由能为根据 Dubinin-Radushkevich 模型,8-12 kJ/mol,证明了所研究的阳离子在 ND 表面微孔上的主要化学吸附机制。超过 10-15 mmol/L 的曲线部分用线性方程描述,Dubinin-Radushkevich 的吸收自由能为 4-6 kJ/mol,被指定为介孔中的物理吸附。开发了一种基于类似的硝酸烧结处理的表面再生程序。与起始材料相比,它提供的吸附参数不会降低;再生过程至少可重复 10 个循环。开发了基于类似的硝酸烧结处理。与起始材料相比,它提供的吸附参数不会降低;再生过程至少可重复 10 个循环。开发了基于类似的硝酸烧结处理。与起始材料相比,它提供的吸附参数不会降低;再生过程至少可重复 10 个循环。
更新日期:2020-12-01
中文翻译:

原始爆炸纳米金刚石作为金属阳离子的可再生吸附剂
摘要 通过从水溶液中吸附镉、锌、镍和铜的例子展示了原始(未通过接枝功能化)爆炸纳米金刚石作为金属阳离子的可逆和重复再生吸附剂的可用性。讨论了各种原始纳米金刚石表面处理。在 250 °C 下用硝酸氧化烧结的过程提供了一个具有更多羧基的表面,对所有测试阳离子的吸附容量都超过 100 μmol/g。测试金属(铜、镍、锌和镉)的吸附等温线可分为两个不同的部分:浓度高达 10-15 mmol/L 的较低部分属于 Giles 的 Langmuir L5 型,吸收自由能为根据 Dubinin-Radushkevich 模型,8-12 kJ/mol,证明了所研究的阳离子在 ND 表面微孔上的主要化学吸附机制。超过 10-15 mmol/L 的曲线部分用线性方程描述,Dubinin-Radushkevich 的吸收自由能为 4-6 kJ/mol,被指定为介孔中的物理吸附。开发了一种基于类似的硝酸烧结处理的表面再生程序。与起始材料相比,它提供的吸附参数不会降低;再生过程至少可重复 10 个循环。开发了基于类似的硝酸烧结处理。与起始材料相比,它提供的吸附参数不会降低;再生过程至少可重复 10 个循环。开发了基于类似的硝酸烧结处理。与起始材料相比,它提供的吸附参数不会降低;再生过程至少可重复 10 个循环。


























 京公网安备 11010802027423号
京公网安备 11010802027423号