当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photochlorination of toluene – the thin line between intensification and selectivity. Part 2: selectivity
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2020-10-7 , DOI: 10.1039/d0re00366b Ümit Taştan 1, 2, 3, 4 , Phillip Seeber 1, 2, 3, 4 , Stephan Kupfer 4, 5, 6, 7 , Dirk Ziegenbalg 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2020-10-7 , DOI: 10.1039/d0re00366b Ümit Taştan 1, 2, 3, 4 , Phillip Seeber 1, 2, 3, 4 , Stephan Kupfer 4, 5, 6, 7 , Dirk Ziegenbalg 1, 2, 3, 4
Affiliation
|
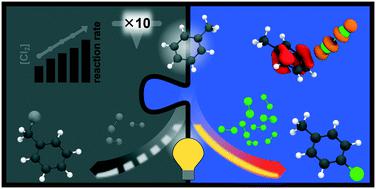
Photochemical reactions, such as the light-driven side-chain chlorination of various aromatic compounds, are among the cornerstones of the chemical industry. Therefore, reoptimising reaction conditions using state-of-the-art reactor designs and irradiation techniques is economically and ecologically of uttermost importance. However, reaction rate enhancement needs to be accompanied by a high selectivity for the desired target compound. In part 1 of this work the potential of intensifying the side-chain photochlorination of toluene by various reactor setups and irradiation techniques was reported. While the reaction was accelerated by up to a factor of ten, its selectivity towards the desired side-chain chlorinated products decreased under certain conditions. Unexpectedly, significant amounts of ring chlorinated species were detected. High level ab initio molecular dynamic simulations allowed – in agreement with the experimental data – assessment of the underlying reaction mechanism leading to the undesired by-product. Photoexcitation of certain toluene–chlorine complexes, formed under highly intensified reaction conditions, yields charge-separated biradical species. In consequence, the pronounced driving force of charge- and radical-recombination leads to the formation of ring chlorinated species as derived by quantum chemical simulations. By applying dynamic irradiation conditions, not only was the formation of ring-chlorinated products suppressed but the energy consumption could also be reduced significantly.
中文翻译:

甲苯的光氯化作用–增强作用与选择性之间的细线。第2部分:选择性
光化学反应,例如各种芳香族化合物的光驱动侧链氯化反应,是化学工业的基础。因此,使用最先进的反应器设计和辐照技术优化反应条件在经济上和生态上都极为重要。但是,反应速率的提高需要伴随着对所需目标化合物的高选择性。在这项工作的第1部分中,报告了通过各种反应器设置和辐照技术增强甲苯侧链光氯化反应的潜力。当反应加速至多十倍时,在某些条件下,其对所需侧链氯化产物的选择性降低。出乎意料的是,检测到大量的环氯化物。高水平从头进行分子动力学模拟-与实验数据一致-可以评估导致不良副产物的潜在反应机理。在高度增强的反应条件下形成的某些甲苯-氯络合物的光激发产生了电荷分离的双自由基物质。结果,明显的电荷和自由基复合驱动力导致形成环氯化物质,这是通过量子化学模拟得出的。通过施加动态辐射条件,不仅可以抑制环氯化产物的形成,而且可以显着降低能耗。
更新日期:2020-12-10
中文翻译:

甲苯的光氯化作用–增强作用与选择性之间的细线。第2部分:选择性
光化学反应,例如各种芳香族化合物的光驱动侧链氯化反应,是化学工业的基础。因此,使用最先进的反应器设计和辐照技术优化反应条件在经济上和生态上都极为重要。但是,反应速率的提高需要伴随着对所需目标化合物的高选择性。在这项工作的第1部分中,报告了通过各种反应器设置和辐照技术增强甲苯侧链光氯化反应的潜力。当反应加速至多十倍时,在某些条件下,其对所需侧链氯化产物的选择性降低。出乎意料的是,检测到大量的环氯化物。高水平从头进行分子动力学模拟-与实验数据一致-可以评估导致不良副产物的潜在反应机理。在高度增强的反应条件下形成的某些甲苯-氯络合物的光激发产生了电荷分离的双自由基物质。结果,明显的电荷和自由基复合驱动力导致形成环氯化物质,这是通过量子化学模拟得出的。通过施加动态辐射条件,不仅可以抑制环氯化产物的形成,而且可以显着降低能耗。

























 京公网安备 11010802027423号
京公网安备 11010802027423号