JACC: Heart Failure ( IF 13.0 ) Pub Date : 2020-10-07 , DOI: 10.1016/j.jchf.2020.08.004 João Pedro Ferreira 1 , Kieran F Docherty 2 , Susan Stienen 3 , Pardeep S Jhund 2 , Brian L Claggett 4 , Scott D Solomon 4 , Mark C Petrie 2 , John Gregson 5 , Stuart J Pocock 5 , Faiez Zannad 6 , John J V McMurray 2
|
Objectives
This study compared ways of describing treatment effects. The objective was to better explain to clinicians and patients what they might expect from a given treatment, not only in terms of relative and absolute risk reduction, but also in projections of long-term survival.
Background
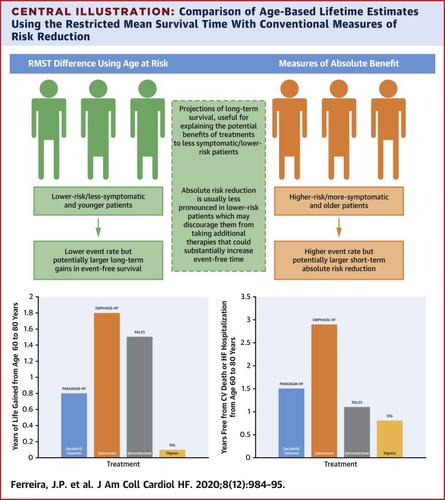
The restricted mean survival time (RMST) can be used to estimate of long-term survival, providing a complementary approach to more conventional metrics (e.g., absolute and relative risk), which may suggest greater benefits of therapy in high-risk patients compared with low-risk patients.
Methods
Relative and absolute risk, as well as the RMST, were calculated in heart failure with reduced ejection fraction (HFrEF) trials.
Results
As examples, in the RALES trial (more severe HFrEF), the treatment effect metrics for spironolactone versus placebo on heart failure hospitalization and/or cardiovascular death were a hazard ratio (HR) of 0.67 (95% confidence interval [CI]: 0.5 to 0.77), number needed to treat = 9 (7 to 14), and age extension of event-free survival +1.1 years (−0.1 to + 2.3 years). The corresponding metrics for EMPHASIS-HF (eplerenone vs. placebo in less severe HFrEF) were 0.64 (0.54 to 0.75), 14 (1 to 22), and +2.9 (1.2 to 4.5). In patients in PARADIGM-HF aged younger than 65 years, the metrics for sacubitril/valsartan versus enalapril were 0.77 (95% CI: 0.68 to 0.88), 23 (15 to 44), and +1.7 (0.6 to 2.8) years; for those aged 65 years or older, the metrics were 0.83 (95% CI: 0.73 to 0.94), 29 (17 to 83), and +0.9 (0.2 to 1.6) years, which provided evidence of a greater potential life extension in younger patients. Similar observations were found for lower risk patients.
Conclusions
RMST event-free (and overall) survival estimates provided a complementary means of evaluating the effect of therapy in relation to age and risk. They also provided a clinically useful metric that should be routinely reported and used to explain the potential long-term benefits of a given treatment, especially to younger and less symptomatic patients.
中文翻译:

估计心力衰竭治疗的终生获益
目标
本研究比较了描述治疗效果的方法。目的是更好地向临床医生和患者解释他们对给定治疗的期望,不仅在相对和绝对风险降低方面,而且在长期生存预测方面。
背景
受限平均生存时间 (RMST) 可用于估计长期生存,为更传统的指标(例如,绝对和相对风险)提供补充方法,这可能表明与高危患者相比,高危患者的治疗获益更大低危患者。
方法
在射血分数降低的心力衰竭 (HFrEF) 试验中计算相对和绝对风险以及 RMST。
结果
例如,在 RALES 试验(更严重的 HFrEF)中,螺内酯与安慰剂对心力衰竭住院和/或心血管死亡的治疗效果指标为风险比 (HR) 为 0.67(95% 置信区间 [CI]:0.5 至0.77),需要治疗的人数 = 9(7 至 14),无事件生存期的年龄延长 +1.1 年(-0.1 至 + 2.3 年)。EMPHASIS-HF(不太严重的 HFrEF 中的依普利农与安慰剂相比)的相应指标为 0.64(0.54 至 0.75)、14(1 至 22)和 +2.9(1.2 至 4.5)。在 PARADIGM-HF 中年龄小于 65 岁的患者中,沙库巴曲/缬沙坦与依那普利的指标分别为 0.77(95% CI:0.68 至 0.88)、23(15 至 44)和 +1.7(0.6 至 2.8)岁;对于 65 岁或以上的人,指标为 0.83(95% CI:0.73 至 0.94)、29(17 至 83)和 +0.9(0.2 至 1.6)岁,这提供了年轻患者更大的潜在寿命延长的证据。对于低风险患者也发现了类似的观察结果。
结论
RMST 无事件(和总体)生存估计提供了一种补充方法来评估与年龄和风险相关的治疗效果。他们还提供了一个临床上有用的指标,应该定期报告并用于解释特定治疗的潜在长期益处,尤其是对年轻和症状较轻的患者。



























 京公网安备 11010802027423号
京公网安备 11010802027423号