当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into the synthesis of N-methylated polypeptides
Polymer Chemistry ( IF 4.6 ) Pub Date : 2020-10-06 , DOI: 10.1039/d0py01055c Christian Muhl 1, 2, 3, 4 , Lydia Zengerling 1, 2, 3, 4 , Jonathan Groß 1, 2, 3, 4 , Paul Eckhardt 1, 2, 3, 4 , Till Opatz 1, 2, 3, 4 , Pol Besenius 1, 2, 3, 4 , Matthias Barz 1, 2, 3, 4, 5
Polymer Chemistry ( IF 4.6 ) Pub Date : 2020-10-06 , DOI: 10.1039/d0py01055c Christian Muhl 1, 2, 3, 4 , Lydia Zengerling 1, 2, 3, 4 , Jonathan Groß 1, 2, 3, 4 , Paul Eckhardt 1, 2, 3, 4 , Till Opatz 1, 2, 3, 4 , Pol Besenius 1, 2, 3, 4 , Matthias Barz 1, 2, 3, 4, 5
Affiliation
|
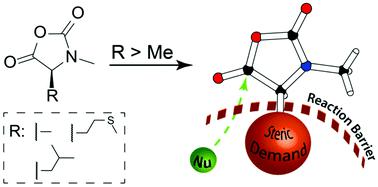
The ring-opening polymerization (ROP) of N-carboxy anhydrides (NCAs) is mostly divided into two classes: NCAs of α-substituted amino acids and N-methylated NCAs of α-unsubstituted glycine derivatives (NNCAs). The use of both monomer types offers different mechanistic features and results in a multitude of functional materials. To combine these properties, the synthesis and ROP of α-substituted and N-methylated NCAs (αNNCAs) of several amino acids were investigated. The current study provides insight into the influence of polymerization conditions and the limitations caused by the enhanced steric demand of the amino acid NCA monomers and their N-methylated derivatives. Namely, the effects of solvent polarity (DMF and DCM) and steric demand of the initiator by using neopentyl amine (NPA) and n-butyl amine (nBu) were studied. Analysis by HFIP-GPC and MALDI-ToF MS reveals that the polymerization and the resulting polymers are tremendously affected by the steric demand of both the initiators and the monomers, while electronic effects seem to have only minor influences. The experimental results are further compared with computational studies, based on coupled cluster (CC) calculations, which underline that electronic effects are of lower importance than steric constraints for the ROP of αNNCAs. Moreover, poly(N-methyl-L-methionine) forms helical secondary structures in solution. Therefore, this work combines mechanistic studies of the ROP of αNNCAs with initial studies on the solution properties of these polypeptides.
中文翻译:

洞察N-甲基化多肽的合成
N-羧酸酐(NCA)的开环聚合(ROP)主要分为两类:α-取代氨基酸的NCA和α-未取代甘氨酸衍生物(NNCA)的N-甲基化NCA。两种单体类型的使用提供了不同的机械特性,并导致了多种功能材料。为了结合这些特性,研究了几种氨基酸的α-取代的和N-甲基化的NCAs(αNNCAs)的合成和ROP 。当前的研究提供了对聚合条件的影响以及氨基酸NCA单体及其N的空间需求增加引起的限制的见解。-甲基化衍生物。即,研究了使用新戊胺(NPA)和正丁胺(n Bu)对溶剂极性(DMF和DCM)和引发剂的空间需求的影响。HFIP-GPC和MALDI-ToF MS的分析表明,引发剂和单体的空间需求极大地影响了聚合反应和所得的聚合物,而电子效应似乎只具有很小的影响。根据耦合簇(CC)计算,将实验结果与计算研究进行了进一步比较,结果表明,对于αNNCA的ROP,电子效应的重要性低于空间约束。此外,聚(N-甲基-L-甲硫氨酸)在溶液中形成螺旋二级结构。因此,这项工作将对αNNCAsROP的机理研究与对这些多肽的溶液性质的初步研究相结合。
更新日期:2020-11-03
中文翻译:

洞察N-甲基化多肽的合成
N-羧酸酐(NCA)的开环聚合(ROP)主要分为两类:α-取代氨基酸的NCA和α-未取代甘氨酸衍生物(NNCA)的N-甲基化NCA。两种单体类型的使用提供了不同的机械特性,并导致了多种功能材料。为了结合这些特性,研究了几种氨基酸的α-取代的和N-甲基化的NCAs(αNNCAs)的合成和ROP 。当前的研究提供了对聚合条件的影响以及氨基酸NCA单体及其N的空间需求增加引起的限制的见解。-甲基化衍生物。即,研究了使用新戊胺(NPA)和正丁胺(n Bu)对溶剂极性(DMF和DCM)和引发剂的空间需求的影响。HFIP-GPC和MALDI-ToF MS的分析表明,引发剂和单体的空间需求极大地影响了聚合反应和所得的聚合物,而电子效应似乎只具有很小的影响。根据耦合簇(CC)计算,将实验结果与计算研究进行了进一步比较,结果表明,对于αNNCA的ROP,电子效应的重要性低于空间约束。此外,聚(N-甲基-L-甲硫氨酸)在溶液中形成螺旋二级结构。因此,这项工作将对αNNCAsROP的机理研究与对这些多肽的溶液性质的初步研究相结合。


























 京公网安备 11010802027423号
京公网安备 11010802027423号