Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The structural basis for signal promiscuity in a bacterial chemoreceptor
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-10-05 , DOI: 10.1111/febs.15580 José Antonio Gavira 1 , Miguel A Matilla 2 , Matilde Fernández 2 , Tino Krell 2
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-10-05 , DOI: 10.1111/febs.15580 José Antonio Gavira 1 , Miguel A Matilla 2 , Matilde Fernández 2 , Tino Krell 2
Affiliation
|
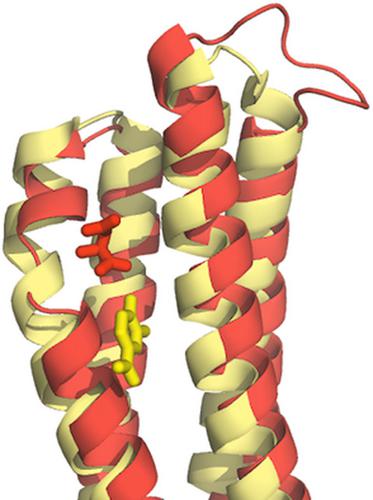
Signalling through chemosensory pathways is typically initiated by the binding of signal molecules to the chemoreceptor ligand binding domain (LBD). The PcaY_PP chemoreceptor from Pseudomonas putida KT2440 is characterized by an unusually broad signal range, and minimal requisites for signal binding are the presence of a C6‐membered ring and that of a carboxyl group. Previous studies have shown that only some of the multiple signals recognized by this chemoreceptor are of apparent metabolic value. We report here high‐resolution structures of PcaY_PP‐LBD in the absence and presence of four cognate chemoeffectors and glycerol. The domain formed a four‐helix bundle (4HB), and both ligand binding sites of the dimer were occupied with the high‐affinity ligands protocatechuate and quinate, whereas the lower‐affinity ligands benzoate and salicylate were present in only one site. Ligand binding was verified by microcalorimetric titration of site‐directed mutants revealing important roles of an arginine and number of polar residues that establish an extensive hydrogen bonding network with bound ligands. The comparison of the apo and holo structures did not provide evidence for this receptor employing a transmembrane signalling mechanism that involves piston‐like shifts of the final helix. Instead, ligand binding caused rigid‐body scissoring movements of both monomers of the dimer. Comparisons with the 4HB domains of the Tar and Tsr chemoreceptors revealed significant structural differences. Importantly, the ligand binding site in PcaY_PP‐LBD is approximately 8 Å removed from that of the Tar and Tsr receptors. Data indicate a significant amount of structural and functional diversity among 4HB domains.
中文翻译:

细菌化学感受器中信号混杂的结构基础
通常通过信号分子与化学感受器配体结合域(LBD)的结合来启动通过化学感应途径发出的信号。恶臭假单胞菌的PcaY_PP化学感受器KT2440具有异常宽广的信号范围,信号结合的最低要求是存在C6元环和羧基。先前的研究表明,该化学感受器识别的多种信号中只有一部分具有明显的代谢价值。我们在此报告了在缺乏和存在四个同源化学效应剂和甘油的情况下PcaY_PP-LBD的高分辨率结构。该结构域形成四螺旋束(4HB),二聚体的两个配体结合位点都被高亲和力的原儿茶酸和奎宁酸占据,而低亲和力的配体苯甲酸盐和水杨酸盐仅存在于一个位点。通过定点突变体的微量量热滴定验证了配体结合,揭示了精氨酸的重要作用和极性残基的数量,这些残基与结合的配体建立了广泛的氢键网络。载脂蛋白和全环结构的比较没有为该受体采用跨膜信号传导机制提供证据,该机制涉及最终螺旋结构的活塞样移动。相反,配体结合导致二聚体两个单体的刚性剪切运动。与Tar和Tsr化学感受器的4HB结构域的比较显示出显着的结构差异。重要的是,PcaY_PP-LBD中的配体结合位点与Tar和Tsr受体的结合位点大约相距8Å。数据表明4HB域之间存在大量的结构和功能多样性。
更新日期:2020-10-05
中文翻译:

细菌化学感受器中信号混杂的结构基础
通常通过信号分子与化学感受器配体结合域(LBD)的结合来启动通过化学感应途径发出的信号。恶臭假单胞菌的PcaY_PP化学感受器KT2440具有异常宽广的信号范围,信号结合的最低要求是存在C6元环和羧基。先前的研究表明,该化学感受器识别的多种信号中只有一部分具有明显的代谢价值。我们在此报告了在缺乏和存在四个同源化学效应剂和甘油的情况下PcaY_PP-LBD的高分辨率结构。该结构域形成四螺旋束(4HB),二聚体的两个配体结合位点都被高亲和力的原儿茶酸和奎宁酸占据,而低亲和力的配体苯甲酸盐和水杨酸盐仅存在于一个位点。通过定点突变体的微量量热滴定验证了配体结合,揭示了精氨酸的重要作用和极性残基的数量,这些残基与结合的配体建立了广泛的氢键网络。载脂蛋白和全环结构的比较没有为该受体采用跨膜信号传导机制提供证据,该机制涉及最终螺旋结构的活塞样移动。相反,配体结合导致二聚体两个单体的刚性剪切运动。与Tar和Tsr化学感受器的4HB结构域的比较显示出显着的结构差异。重要的是,PcaY_PP-LBD中的配体结合位点与Tar和Tsr受体的结合位点大约相距8Å。数据表明4HB域之间存在大量的结构和功能多样性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号