Journal of King Saud University-Science ( IF 3.8 ) Pub Date : 2020-10-06 , DOI: 10.1016/j.jksus.2020.09.026 Ramesh S Gani , Karabasanagouda Timanagouda , S. Madhushree , Shrinivas D Joshi , Murigendra B. Hiremath , Salma Begum Hussain Mujawar , Avinash Kundadka Kudva
|
Objective
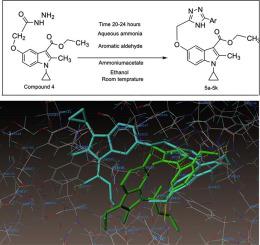
In the present study a series of eleven bis-heterocyclic compounds with indole derivative carrying 1,2,4-triazole moiety were synthesized and assessed for their in vitro α-amylase and α-glucosidase inhibition activity.
Method
The synthesized compounds were characterized by using various spectroscopic techniques such as 1H NMR, IR and EI-MS. Initial in silico screening process was used to find potential ligands that were later evaluated for α-amylase and α-glucosidase inhibitory potential.
Results
The docking results revealed that the synthesized compounds were well accommodated in the binding pockets of α-glucosidase. Especially, 5e and 5j showed similar interaction pattern, as previously reported Casuarine-enzyme complex. In vitro analysis suggests that compounds 5a-5k showed varying degrees of α-amylase and α-glucosidase inhibitory activity. Amongst them, 5e and 5j demonstrated good enzyme inhibition while remaining compounds showed low to moderate inhibitory potential.
Conclusions
Addition of 2,5 dimethoxy substituent (2,5-dimethoxybenzaldehyde) (5e) or hydroxy, methoxy substituents (6-methoxy-2-naphthol aldehyde) (5j) at ortho and meta position exhibited good α-amylase and α-glucosidase inhibition. Hence this study provides several insights on improving the pharmacological profile of triazole containing compounds that can be adopted to design and develop novel glucosidase inhibitors.
中文翻译:

新型吲哚1,2,4-三唑衍生物作为潜在的葡糖苷酶抑制剂的合成
目的
在本研究中,合成了十一种带有带有1,2,4-三唑部分的吲哚衍生物的双杂环化合物,并对其体外α-淀粉酶和α-葡萄糖苷酶的抑制活性进行了评估。
方法
通过使用各种光谱技术,如1 H NMR,IR和EI-MS对合成的化合物进行表征。最初的计算机筛选过程用于寻找潜在的配体,随后评估其对α-淀粉酶和α-葡萄糖苷酶的抑制潜力。
结果
对接结果表明,合成的化合物很好地容纳在α-葡萄糖苷酶的结合口袋中。特别是,5e和5j表现出相似的相互作用模式,如先前报道的Casuarine-酶复合物。体外分析表明,化合物5a-5k显示出不同程度的α-淀粉酶和α-葡萄糖苷酶抑制活性。其中5e和5j表现出良好的酶抑制作用,而其余化合物则表现出低至中等的抑制潜力。
结论
在邻位和间位添加2,5二甲氧基取代基(2,5-二甲氧基苯甲醛)(5e)或羟基,甲氧基取代基(6-甲氧基-2-萘醛)(5j)表现出良好的α-淀粉酶和α-葡萄糖苷酶抑制作用。因此,本研究为改善含三唑化合物的药理作用提供了一些见识,这些化合物可用于设计和开发新型葡糖苷酶抑制剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号