Physica E: Low-dimensional Systems and Nanostructures ( IF 3.3 ) Pub Date : 2020-10-06 , DOI: 10.1016/j.physe.2020.114483 Md. Rakib Hossain , Md. Mehade Hasan , Noor-E- Ashrafi , Hamidur Rahman , Mohammad Sadiqur Rahman , Farid Ahmed , Tahmina Ferdous , Md. Abul Hossain
|
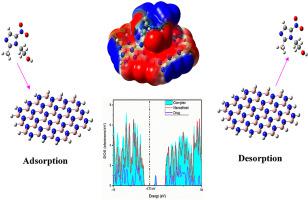
In this present study, we have theoretically scrutinized the interaction mechanism of Metronidazole drug on the surface of graphene, boron carbide and boron nitride nanosheets in gas and aqueous phase applying density functional theory with B3LYP/6-31G method to sort a path for minimizing the adverse effects of this drug on the human and animal body. The maximum adsorption energy (A.E) value is −44.50 kj/mol which indicates the adsorption of all complexes is physisorption type and our complexes benefit from the short recovery time. Weak electrostatic nature of the interaction is also revealed by the QTAIM study. The reduction of Eg for the adsorption of ML on BN sheet is the highest (up to 55%) indicating the highest sensitivity while the sensitivity trend is σ (BN) > σ (GNS) > σ (BC3). The UV–Vis spectra investigation also validates this sensitivity trend. The dipole moment and A.E data in case of the solvent effect upon the complexes claim the favourable adsorption process in aqueous medium. Consequently, this comparative study reveals the ability of BN nanosheet to sense ML drug is higher than that of GNS and BC3, which will help to protect human and wildlife from the side effect of this drug.
中文翻译:

甲硝唑药物分子在氢化石墨烯,氮化硼和碳化硼纳米片在气体和水介质中的表面吸附行为:DFT和QTAIM的比较
在本研究中,我们从理论上详细研究了甲硝唑药物在气相和水相中在石墨烯,碳化硼和氮化硼纳米片表面上的相互作用机理,并运用B3LYP / 6-31G方法通过密度泛函理论为最大限度地减少了该药物对人体和动物体的不良影响。最大吸附能(AE)值为-44.50 kj / mol,这表明所有配合物的吸附均为物理吸附类型,我们的配合物受益于较短的回收时间。QTAIM研究还显示了相互作用的弱静电性质。E g的减少ML在BN片材上的吸附最高(最高55%)表明灵敏度最高,而灵敏度趋势为σ(BN)>σ(GNS)>σ(BC3)。UV-Vis光谱研究也验证了这种敏感性趋势。在溶剂对配合物有影响的情况下,偶极矩和AE数据要求在水性介质中具有良好的吸附过程。因此,该比较研究表明,BN纳米片感知ML药物的能力高于GNS和BC3,这将有助于保护人类和野生生物免受该药物的副作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号