当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective oxidation of alkyl and aryl glyceryl monoethers catalysed by an engineered and immobilised glycerol dehydrogenase
Chemical Science ( IF 8.4 ) Pub Date : 2020-10-05 , DOI: 10.1039/d0sc04471g Susana Velasco-Lozano 1, 2, 3, 4, 5 , Maite Roca 5, 6, 7, 8 , Alejandro Leal-Duaso 1, 2, 3, 4, 5 , José A. Mayoral 1, 2, 3, 4, 5 , Elisabet Pires 1, 2, 3, 4, 5 , Vicent Moliner 5, 6, 7, 8 , Fernando López-Gallego 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2020-10-05 , DOI: 10.1039/d0sc04471g Susana Velasco-Lozano 1, 2, 3, 4, 5 , Maite Roca 5, 6, 7, 8 , Alejandro Leal-Duaso 1, 2, 3, 4, 5 , José A. Mayoral 1, 2, 3, 4, 5 , Elisabet Pires 1, 2, 3, 4, 5 , Vicent Moliner 5, 6, 7, 8 , Fernando López-Gallego 1, 2, 3, 4, 5
Affiliation
|
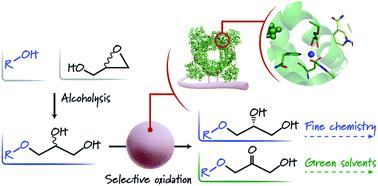
Enzymes acting over glyceryl ethers are scarce in living cells, and consequently biocatalytic transformations of these molecules are rare despite their interest for industrial chemistry. In this work, we have engineered and immobilised a glycerol dehydrogenase from Bacillus stearothermophilus (BsGlyDH) to accept a battery of alkyl/aryl glyceryl monoethers and catalyse their enantioselective oxidation to yield the corresponding 3-alkoxy/aryloxy-1-hydroxyacetones. QM/MM computational studies decipher the key role of D123 in the oxidation catalytic mechanism, and reveal that this enzyme is highly enantioselective towards S-isomers (ee > 99%). Through structure-guided site-selective mutagenesis, we find that the mutation L252A sculpts the active site to accommodate a productive configuration of 3-monoalkyl glycerols. This mutation enhances the kcat 163-fold towards 3-ethoxypropan-1,2-diol, resulting in a specific activity similar to the one found for the wild-type towards glycerol. Furthermore, we immobilised the L252A variant to intensify the process, demonstrating the reusability and increasing the operational stability of the resulting heterogeneous biocatalyst. Finally, we manage to integrate this immobilised enzyme into a one-pot chemoenzymatic process to convert glycidol and ethanol into 3-ethoxy-1-hydroxyacetone and (R)-3-ethoxypropan-1,2-diol, without affecting the oxidation activity. These results thus expand the uses of engineered glycerol dehydrogenases in applied biocatalysis for the kinetic resolution of glycerol ethers and the manufacturing of substituted hydroxyacetones.
中文翻译:

通过工程化和固定化的甘油脱氢酶催化烷基和芳基甘油单醚的选择性氧化
作用于甘油醚的酶在活细胞中是稀缺的,因此尽管它们对工业化学很感兴趣,但是这些分子的生物催化转化却很少。在这项工作中,我们设计并固定了嗜热脂肪芽孢杆菌(BsGlyDH)的甘油脱氢酶,以接受一系列烷基/芳基甘油单醚并催化其对映选择性氧化,从而生成相应的3-烷氧基/芳氧基-1-羟基丙酮。QM / MM计算研究破译了D123在氧化催化机理中的关键作用,并揭示了该酶对S具有高度对映选择性-异构体(ee> 99%)。通过结构指导的位点选择性诱变,我们发现突变L252A雕刻了活性位点,以适应3-单烷基甘油的生产型构型。这一突变使k cat向3-乙氧基丙烷-1,2-二醇的方向增强了163倍,从而产生了一种类似于野生型针对甘油的特异活性。此外,我们固定化了L252A变体以增强该过程,证明了所得异质生物催化剂的可重复使用性并提高了其运行稳定性。最后,我们设法此固定化酶成一锅化学酶促过程转换缩水甘油和乙醇集成到3-乙氧基1-羟基丙酮和(ř)-3-乙氧基丙烷-1,2-二醇,而不影响氧化活性。因此,这些结果扩大了工程甘油脱氢酶在应用生物催化中用于甘油醚的动力学拆分和制备取代的羟基丙酮的用途。
更新日期:2020-10-14
中文翻译:

通过工程化和固定化的甘油脱氢酶催化烷基和芳基甘油单醚的选择性氧化
作用于甘油醚的酶在活细胞中是稀缺的,因此尽管它们对工业化学很感兴趣,但是这些分子的生物催化转化却很少。在这项工作中,我们设计并固定了嗜热脂肪芽孢杆菌(BsGlyDH)的甘油脱氢酶,以接受一系列烷基/芳基甘油单醚并催化其对映选择性氧化,从而生成相应的3-烷氧基/芳氧基-1-羟基丙酮。QM / MM计算研究破译了D123在氧化催化机理中的关键作用,并揭示了该酶对S具有高度对映选择性-异构体(ee> 99%)。通过结构指导的位点选择性诱变,我们发现突变L252A雕刻了活性位点,以适应3-单烷基甘油的生产型构型。这一突变使k cat向3-乙氧基丙烷-1,2-二醇的方向增强了163倍,从而产生了一种类似于野生型针对甘油的特异活性。此外,我们固定化了L252A变体以增强该过程,证明了所得异质生物催化剂的可重复使用性并提高了其运行稳定性。最后,我们设法此固定化酶成一锅化学酶促过程转换缩水甘油和乙醇集成到3-乙氧基1-羟基丙酮和(ř)-3-乙氧基丙烷-1,2-二醇,而不影响氧化活性。因此,这些结果扩大了工程甘油脱氢酶在应用生物催化中用于甘油醚的动力学拆分和制备取代的羟基丙酮的用途。


























 京公网安备 11010802027423号
京公网安备 11010802027423号