当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Optimized Sample Preparation and Data Processing of Data-Independent Acquisition Methods for the Robust Quantification of Trace-Level Host Cell Protein Impurities in Antibody Drug Products
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-10-04 , DOI: 10.1021/acs.jproteome.0c00664 Nicolas Pythoud 1 , Joanna Bons 1 , Geoffroy Mijola 2 , Alain Beck 2 , Sarah Cianférani 1 , Christine Carapito 1
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-10-04 , DOI: 10.1021/acs.jproteome.0c00664 Nicolas Pythoud 1 , Joanna Bons 1 , Geoffroy Mijola 2 , Alain Beck 2 , Sarah Cianférani 1 , Christine Carapito 1
Affiliation
|
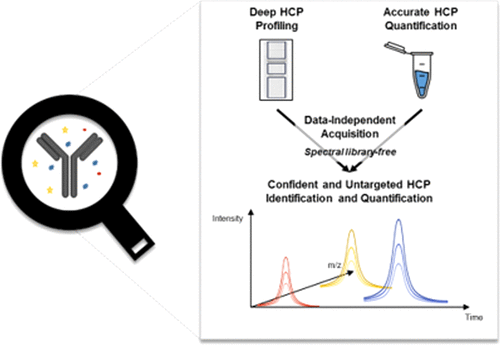
Host cell proteins (HCPs) are a major class of bioprocess-related impurities generated by the host organism and are generally present at low levels in purified biopharmaceutical products. The monitoring of these impurities is identified as an important critical quality attribute of monoclonal antibody (mAb) formulations not only due to the potential risk for the product stability and efficacy but also concerns linked to the immunogenicity of some of them. While overall HCP levels are usually monitored by enzyme-linked immunosorbent assay (ELISA), mass spectrometry (MS)-based approaches have been emerging as powerful and promising alternatives providing qualitative and quantitative information. However, a major challenge for liquid chromatography (LC)-MS-based methods is to deal with the wide dynamic range of drug products and the extreme sensitivity required to detect trace-level HCPs. In this study, we developed powerful and reproducible MS-based analytical workflows coupling optimized and efficient sample preparations, the library-free data-independent acquisition (DIA) method, and stringent validation criteria. The performances of several preparation protocols and DIA versus classical data-dependent acquisition (DDA) were evaluated using a series of four commercially available drug products. Depending on the selected protocols, the user has access to different information: on the one hand, a deep profiling of tens of identified HCPs and on the other hand, accurate and reproducible (coefficients of variation (CVs) < 12%) quantification of major HCPs. Overall, a final global HCP amount of a few tens of ng/mg mAb in these mAb samples was measured, while reaching a sensitivity down to the sub-ng/mg mAb level. Thus, this straightforward and robust approach can be intended as a routine quality control for any drug product analysis.
中文翻译:

抗体药物产品中痕量级宿主细胞蛋白质杂质的稳健定量的数据独立采集方法的优化样品制备和数据处理
宿主细胞蛋白(HCP)是宿主生物产生的与生物过程相关的主要杂质,通常在纯化的生物制药产品中含量较低。这些杂质的监测被确定为单克隆抗体(mAb)制剂的重要重要质量属性,这不仅是因为产品稳定性和功效存在潜在风险,而且还涉及其中某些物质的免疫原性。虽然通常通过酶联免疫吸附测定(ELISA)监控总体HCP水平,但基于质谱(MS)的方法已成为提供定性和定量信息的强大而有前途的替代方法。然而,基于液相色谱(LC)-MS的方法面临的主要挑战是应对宽范围的药品动态范围以及检测痕量HCP所需的极高灵敏度。在这项研究中,我们开发了功能强大且可重现的基于MS的分析工作流程,结合了优化和高效的样品制备,无库数据独立获取(DIA)方法以及严格的验证标准。使用一系列四种市售药品评估了几种制备方案的性能,以及DIA与经典数据依赖采集(DDA)的性能。根据选择的协议,用户可以访问不同的信息:一方面,对数十种已识别的HCP进行深度分析,另一方面,准确且可重现(变异系数(CV)< 12%)量化主要HCP。总体而言,在这些mAb样品中,最终总体HCP量为几十ng / mg mAb,同时灵敏度低至亚ng / mg mAb水平。因此,这种简单而稳健的方法可以用作任何药品分析的常规质量控制。
更新日期:2020-10-04
中文翻译:

抗体药物产品中痕量级宿主细胞蛋白质杂质的稳健定量的数据独立采集方法的优化样品制备和数据处理
宿主细胞蛋白(HCP)是宿主生物产生的与生物过程相关的主要杂质,通常在纯化的生物制药产品中含量较低。这些杂质的监测被确定为单克隆抗体(mAb)制剂的重要重要质量属性,这不仅是因为产品稳定性和功效存在潜在风险,而且还涉及其中某些物质的免疫原性。虽然通常通过酶联免疫吸附测定(ELISA)监控总体HCP水平,但基于质谱(MS)的方法已成为提供定性和定量信息的强大而有前途的替代方法。然而,基于液相色谱(LC)-MS的方法面临的主要挑战是应对宽范围的药品动态范围以及检测痕量HCP所需的极高灵敏度。在这项研究中,我们开发了功能强大且可重现的基于MS的分析工作流程,结合了优化和高效的样品制备,无库数据独立获取(DIA)方法以及严格的验证标准。使用一系列四种市售药品评估了几种制备方案的性能,以及DIA与经典数据依赖采集(DDA)的性能。根据选择的协议,用户可以访问不同的信息:一方面,对数十种已识别的HCP进行深度分析,另一方面,准确且可重现(变异系数(CV)< 12%)量化主要HCP。总体而言,在这些mAb样品中,最终总体HCP量为几十ng / mg mAb,同时灵敏度低至亚ng / mg mAb水平。因此,这种简单而稳健的方法可以用作任何药品分析的常规质量控制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号