当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental Liquid–Liquid Equilibrium and Solubility Study of an Acetic Acid–n-Propyl Alcohol–n-Propyl Acetate–Water System at 323.15 and 333.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-10-05 , DOI: 10.1021/acs.jced.0c00501 Maria Toikka 1 , Alexey Sadaev 1 , Olga Lobacheva 2 , Alexandra Golikova 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-10-05 , DOI: 10.1021/acs.jced.0c00501 Maria Toikka 1 , Alexey Sadaev 1 , Olga Lobacheva 2 , Alexandra Golikova 1
Affiliation
|
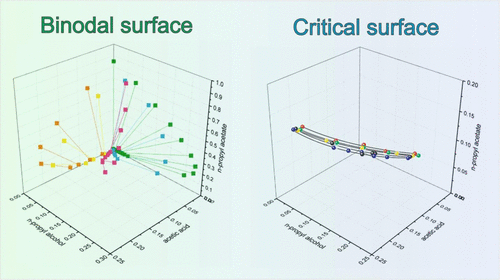
Liquid–liquid equilibrium (LLE) and solubility for the quaternary acetic acid–n-propyl alcohol–n-propyl acetate–water were carried out at 323.15 and 333.15 K (atmospheric pressure). A brief review of literature data is provided. The compositions of LLE were determined by gas chromatography analysis. Solubility data and critical points were obtained by the “cloud-point” technique method. The obtained data gave an opportunity to present the disposition of the critical curve formed by critical points and binodal surface in a regular composition tetrahedron. A set of critical curves led to a critical polythermal surface in the 3D concentration space. A detailed graphical analysis of data comparison is presented for binary and ternary subsystems. LLE modeling using the NRTL model shows that calculated data are in good agreement with the experimental results.
中文翻译:

实验液-液平衡和乙酸-的溶解度研究ñ丙基酒精ň乙酸异丙酯-水体系在323.15和333.15ķ
季乙酸–正丙醇– n的液-液平衡(LLE)和溶解度乙酸丙酯-水在323.15和333.15 K(大气压)下进行。提供了文献数据的简要回顾。通过气相色谱分析确定LLE的组成。溶解度数据和临界点通过“浊点”技术方法获得。获得的数据提供了一个机会,可以介绍由规则点和四面体组成的临界点和二面体表面形成的临界曲线的位置。一组关键曲线导致3D集中空间中出现了关键的多热表面。给出了二进制和三进制子系统的数据比较的详细图形分析。使用NRTL模型的LLE建模表明,计算数据与实验结果非常吻合。
更新日期:2020-11-12
中文翻译:

实验液-液平衡和乙酸-的溶解度研究ñ丙基酒精ň乙酸异丙酯-水体系在323.15和333.15ķ
季乙酸–正丙醇– n的液-液平衡(LLE)和溶解度乙酸丙酯-水在323.15和333.15 K(大气压)下进行。提供了文献数据的简要回顾。通过气相色谱分析确定LLE的组成。溶解度数据和临界点通过“浊点”技术方法获得。获得的数据提供了一个机会,可以介绍由规则点和四面体组成的临界点和二面体表面形成的临界曲线的位置。一组关键曲线导致3D集中空间中出现了关键的多热表面。给出了二进制和三进制子系统的数据比较的详细图形分析。使用NRTL模型的LLE建模表明,计算数据与实验结果非常吻合。

























 京公网安备 11010802027423号
京公网安备 11010802027423号