当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Dehydrogenation of Ammonia Borane Mediated by a Pt(0)/Borane Frustrated Lewis Pair: Theoretical Design
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-10-04 , DOI: 10.1002/cphc.202000661 Lei Zhang 1 , Takumi Oishi 2 , Liuzhou Gao 3 , Shiyu Hu 3 , Linlin Yang 1 , Wei Li 3 , Shengjun Wu 1 , Rong Shang 2 , Yohsuke Yamamoto 2 , Shuhua Li 3 , Wei Wang 1 , Guixiang Zeng 4
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-10-04 , DOI: 10.1002/cphc.202000661 Lei Zhang 1 , Takumi Oishi 2 , Liuzhou Gao 3 , Shiyu Hu 3 , Linlin Yang 1 , Wei Li 3 , Shengjun Wu 1 , Rong Shang 2 , Yohsuke Yamamoto 2 , Shuhua Li 3 , Wei Wang 1 , Guixiang Zeng 4
Affiliation
|
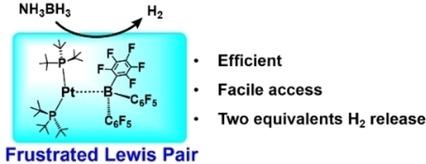
A new efficient metal‐based frustrated Lewis pair constructed by (PtBu3)2Pt and B(C6F5)3 was designed through density functional theory calculations for the catalytic dehydrogenation of ammonia borane (AB). The reaction was composed by the successive dehydrogenation of AB and H2 liberation, which occurs through the cooperative functions of the Pt(0) center and the B(C6F5)3 moiety. Two equivalents of H2 were predicted to be liberated from each AB molecule. The generation of the second H2 is the rate‐determining step, with a Gibbs energy barrier and reaction energy of 27.4 and 12.8 kcal/mol, respectively.
中文翻译:

Pt(0)/硼烷失衡的路易斯对介导的氨硼烷的催化脱氢:理论设计
通过密度泛函理论计算,设计了一种新的由(P t Bu 3)2 Pt和B(C 6 F 5)3构成的新型金属基路易斯化对,用于氨硼烷(AB)的催化脱氢。该反应由AB的连续脱氢和H 2的释放组成,这是通过Pt(0)中心和B(C 6 F 5)3部分的协同功能发生的。H 2的两个当量预计会从每个AB分子中释放出来。第二个H2的生成是速率确定步骤,吉布斯能垒和反应能分别为27.4和12.8 kcal / mol。
更新日期:2020-12-02
中文翻译:

Pt(0)/硼烷失衡的路易斯对介导的氨硼烷的催化脱氢:理论设计
通过密度泛函理论计算,设计了一种新的由(P t Bu 3)2 Pt和B(C 6 F 5)3构成的新型金属基路易斯化对,用于氨硼烷(AB)的催化脱氢。该反应由AB的连续脱氢和H 2的释放组成,这是通过Pt(0)中心和B(C 6 F 5)3部分的协同功能发生的。H 2的两个当量预计会从每个AB分子中释放出来。第二个H2的生成是速率确定步骤,吉布斯能垒和反应能分别为27.4和12.8 kcal / mol。



























 京公网安备 11010802027423号
京公网安备 11010802027423号