Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-10-05 , DOI: 10.1016/j.jmb.2020.09.013 Aditya J Basak 1 , Snigdha Maiti 1 , Anita Hansda 2 , Dhrubajyoti Mahata 3 , Kheerthana Duraivelan 1 , Shankar V Kundapura 4 , Woonghee Lee 5 , Gayatri Mukherjee 2 , Soumya De 1 , Dibyendu Samanta 1
|
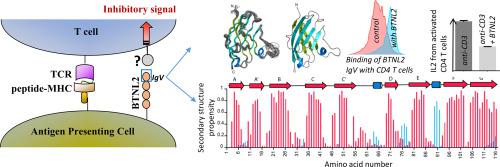
T cell costimulation is mediated by the interaction of a number of receptors and ligands present on the surface of the T cell and antigen-presenting cell, respectively. Stimulatory or inhibitory signals from these receptor–ligand interactions work in tandem to preserve immune homeostasis. BTNL2 is a type-1 membrane protein that provides inhibitory signal to T cells and plays an important role in several inflammatory and autoimmune diseases. Therefore, manipulation of the molecular interaction of BTNL2 with its putative receptor could provide strategies to restore immune homeostasis in these diseases. Hence, it is imperative to study the structural characteristics of this molecule, which will provide important insights into its function as well. In this study, the membrane-distal ectodomain of murine BTNL2 was expressed in bacteria as inclusion bodies, refolded in vitro and purified for functional and structural characterization. The domain is monomeric in solution as demonstrated by size-exclusion chromatography and analytical ultracentrifugation, and also binds to its putative receptor on naïve B cells and activated T cell subsets. Importantly, for the first time, we report the structure of BTNL2 as determined by solution NMR spectroscopy and also the picosecond–nanosecond timescale backbone dynamics of this domain. The N-terminal ectodomain of BTNL2, which was able to inhibit T cell function as well, exhibits distinctive structural features. The N-terminal ectodomain of BTNL2 has a significantly reduced surface area in the front sheet due to the non-canonical conformation of the CC′ loop, which provides important insights into the recognition of its presently unknown binding partner.
中文翻译:

BTNL2 的 N 端 IgV 结构域的结构洞察,一种 T 细胞抑制分子,建议其推定受体的非规范结合界面
T 细胞共刺激由分别存在于 T 细胞和抗原呈递细胞表面的多种受体和配体的相互作用介导。来自这些受体-配体相互作用的刺激或抑制信号协同工作以保持免疫稳态。BTNL2 是一种 1 型膜蛋白,可为 T 细胞提供抑制信号,并在多种炎症和自身免疫疾病中发挥重要作用。因此,操纵 BTNL2 与其假定受体的分子相互作用可以提供在这些疾病中恢复免疫稳态的策略。因此,研究该分子的结构特征势在必行,这也将为其功能提供重要的见解。在这项研究中,体外并纯化用于功能和结构表征。如尺寸排阻色谱和分析超速离心所证明的,该结构域在溶液中是单体的,并且还与其在幼稚 B 细胞和活化 T 细胞亚群上的推定受体结合。重要的是,我们首次报告了由溶液核磁共振光谱确定的 BTNL2 结构以及该域的皮秒-纳秒时间尺度骨架动力学。BTNL2 的 N 端胞外域也能够抑制 T 细胞功能,表现出独特的结构特征。由于 CC' 环的非规范构象,BTNL2 的 N 端胞外域在前片中的表面积显着减少,这为识别其目前未知的结合伴侣提供了重要的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号