当前位置:
X-MOL 学术
›
Can. J. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparative thermodynamic analysis of CO2 based dimethyl carbonate synthesis routes
The Canadian Journal of Chemical Engineering ( IF 2.1 ) Pub Date : 2020-10-03 , DOI: 10.1002/cjce.23893 Shailesh Pandey 1 , Vimal Chandra Srivastava 1 , Vimal Kumar 1
The Canadian Journal of Chemical Engineering ( IF 2.1 ) Pub Date : 2020-10-03 , DOI: 10.1002/cjce.23893 Shailesh Pandey 1 , Vimal Chandra Srivastava 1 , Vimal Kumar 1
Affiliation
|
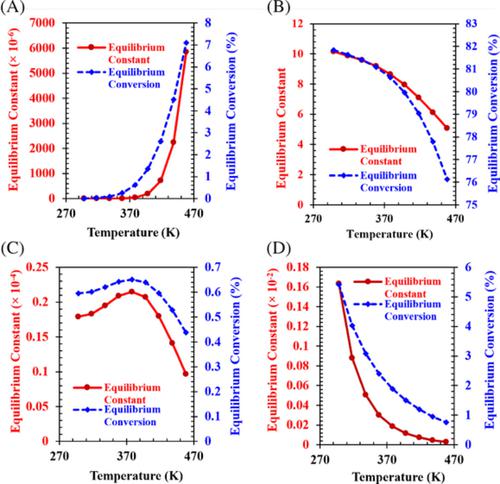
The chemical equilibrium analysis of various possible reactions involved in the five CO2‐based routes for the synthesis of dimethyl carbonate (DMC), a green chemical reagent, was performed in the present study. The variation of the molar Gibbs free energy change with temperature for the major reactions of DMC synthesis in all five routes was studied in the specified temperature range of 298.15 K‐453.15 K and at both 1 bar and 28 bar pressures (1 bar = 105 Pa) to observe the thermodynamic favourability of all routes. To observe the temperature effect, the molar heat capacities for various compounds were estimated in terms of temperature. A second‐order group additivity method called the Ruzicka‐Domalski method was used to estimate these heat capacity values. The major reactions of DMC synthesis in urea methanolysis, EC transesterification, PC transesterification, and direct CO2 synthesis routes were compared by investigating the effect of temperature (in the range of 298.15 K‐453.15 K) on the chemical equilibrium constants and the equilibrium conversions of methanol at a constant pressure of 28 bar. The one‐pot propylene oxide (PO) synthesis route was observed to be better among all the five CO2 based routes when it was compared with respect to values of the chemical equilibrium constant and equilibrium conversion of methanol at standard conditions. The reliability of the EC transesterification route was found better among all the routes at initial conditions (T = 298.15 K; P = 28 bar). While the urea methanolysis route was found as least favourable at initial conditions.
中文翻译:

二氧化碳基碳酸二甲酯合成路线的比较热力学分析
在本研究中,对五种基于CO 2的路线合成绿色化学试剂碳酸二甲酯(DMC)涉及的各种可能的反应进行了化学平衡分析。在指定的温度范围298.15 K-453.15 K以及1 bar和28 bar的压力下(1 bar = 10 5),研究了五种途径中DMC合成主要反应的摩尔吉布斯自由能随温度的变化。 Pa)观察所有路线的热力学偏好。为了观察温度的影响,根据温度估算了各种化合物的摩尔热容。使用一种称为Ruzicka-Domalski方法的二阶组可加性方法来估算这些热容值。通过研究温度(在298.15 K-453.15 K范围内)对化学平衡常数和平衡转化率的影响,比较了DMC合成在尿素甲醇分解,EC酯交换,PC酯交换和直接CO 2合成路线中的主要反应。在28 bar的恒定压力下使用甲醇。在所有五个CO 2中,单锅环氧丙烷(PO)的合成路线均被认为更好相对于标准条件下甲醇的化学平衡常数和平衡转化率的值进行比较时,可以选择不同的路线。在初始条件下(T = 298.15 K;P = 28 bar),所有路线中的EC酯交换路线的可靠性都更高。虽然在初始条件下发现尿素甲醇分解路线最不利。
更新日期:2020-10-03
中文翻译:

二氧化碳基碳酸二甲酯合成路线的比较热力学分析
在本研究中,对五种基于CO 2的路线合成绿色化学试剂碳酸二甲酯(DMC)涉及的各种可能的反应进行了化学平衡分析。在指定的温度范围298.15 K-453.15 K以及1 bar和28 bar的压力下(1 bar = 10 5),研究了五种途径中DMC合成主要反应的摩尔吉布斯自由能随温度的变化。 Pa)观察所有路线的热力学偏好。为了观察温度的影响,根据温度估算了各种化合物的摩尔热容。使用一种称为Ruzicka-Domalski方法的二阶组可加性方法来估算这些热容值。通过研究温度(在298.15 K-453.15 K范围内)对化学平衡常数和平衡转化率的影响,比较了DMC合成在尿素甲醇分解,EC酯交换,PC酯交换和直接CO 2合成路线中的主要反应。在28 bar的恒定压力下使用甲醇。在所有五个CO 2中,单锅环氧丙烷(PO)的合成路线均被认为更好相对于标准条件下甲醇的化学平衡常数和平衡转化率的值进行比较时,可以选择不同的路线。在初始条件下(T = 298.15 K;P = 28 bar),所有路线中的EC酯交换路线的可靠性都更高。虽然在初始条件下发现尿素甲醇分解路线最不利。



























 京公网安备 11010802027423号
京公网安备 11010802027423号