当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting the Rich Conformational Landscape of N‐Allylmethylamine Using Rotational Spectroscopy and Quantum Mechanical Calculations
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-10-03 , DOI: 10.1002/cphc.202000757 Weslley G. D. P. Silva 1 , Tamanna Poonia 2 , Jennifer van Wijngaarden 3
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-10-03 , DOI: 10.1002/cphc.202000757 Weslley G. D. P. Silva 1 , Tamanna Poonia 2 , Jennifer van Wijngaarden 3
Affiliation
|
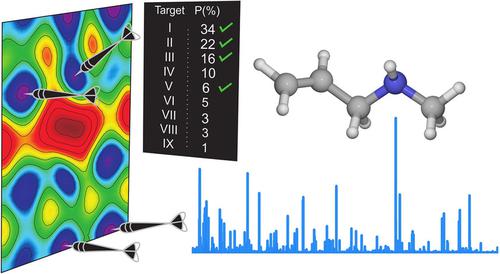
The highly variable conformational landscape of N‐allylmethylamine (AMA) was investigated using Fourier transform microwave spectroscopy aided by high‐level theoretical calculations to understand the energy relationship governing the interconversion between nine stable conformers. Spectroscopically, transitions belonging to four low energy conformers were identified and their hyperfine patterns owing to the 14N quadrupolar nucleus were unambiguously resolved. The rotational spectrum of the global minimum geometry, conformer I, shows an additional splitting associated with a tunneling motion through an energy barrier interconnecting its enantiomeric forms. A two‐step tunneling trajectory is proposed by finding transition state structures corresponding to the allyl torsion and NH inversion. Natural bond orbital and non‐covalent interaction analyses reveal that an interplay between steric and hyperconjugative effects rules the conformational preferences of AMA.
中文翻译:

使用旋转光谱法和量子力学计算确定N-烯丙基甲胺的丰富构象景观
N-烯丙基甲胺(AMA)的高度可变的构象景观是使用傅立叶变换微波光谱技术借助高级理论计算来研究的,以了解控制9个稳定构象异构体之间相互转化的能量关系。在光谱学上,鉴定了属于四个低能构象异构体的跃迁,并归因于14个N四极核被明确分辨。整体最小几何形状的构象异构体I的旋转光谱显示了与通过穿过其对映体形式相互连接的能垒的隧穿运动相关的附加分裂。通过寻找对应于烯丙基扭转和NH反型的过渡态结构,提出了两步隧穿轨迹。自然键轨道和非共价相互作用分析表明,空间和超共轭效应之间的相互作用决定了AMA的构象偏好。
更新日期:2020-11-18
中文翻译:

使用旋转光谱法和量子力学计算确定N-烯丙基甲胺的丰富构象景观
N-烯丙基甲胺(AMA)的高度可变的构象景观是使用傅立叶变换微波光谱技术借助高级理论计算来研究的,以了解控制9个稳定构象异构体之间相互转化的能量关系。在光谱学上,鉴定了属于四个低能构象异构体的跃迁,并归因于14个N四极核被明确分辨。整体最小几何形状的构象异构体I的旋转光谱显示了与通过穿过其对映体形式相互连接的能垒的隧穿运动相关的附加分裂。通过寻找对应于烯丙基扭转和NH反型的过渡态结构,提出了两步隧穿轨迹。自然键轨道和非共价相互作用分析表明,空间和超共轭效应之间的相互作用决定了AMA的构象偏好。



























 京公网安备 11010802027423号
京公网安备 11010802027423号