当前位置:
X-MOL 学术
›
Surf. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
MgO nano-sheets for adsorption of anionic dyes from aqueous solution: Equilibrium and kinetics studies
Surfaces and Interfaces ( IF 6.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.surfin.2020.100722 Reza Dalvand , Effat Kianpour , Haniyeh Tahzibi , Saeid Azizian
Surfaces and Interfaces ( IF 6.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.surfin.2020.100722 Reza Dalvand , Effat Kianpour , Haniyeh Tahzibi , Saeid Azizian
|
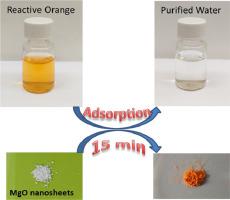
Abstract MgO nano-sheets with a thickness of 26 nm and reticular structure were synthesized and characterized using FE-SEM, XRD, EDX and N2 adsorption/desorption. The synthesized MgO nano-sheets were used to remove two anionic dye pollutants (reactive orange and reactive yellow) from aqueous solution. The equilibrium and kinetics data were obtained, and fitted very well with Langmuir-Freundlich and Redlich-Peterson isotherms, and fractal like-pseudo second order kinetics model. The effect of solution pH and temperature on the removal efficiency of the synthesized adsorbent was studied in details. The maximum adsorption capacity of MgO nano-sheets to remove reactive orange is more than 30 times higher than reactive yellow. The difference in the adsorption mechanisms is proposed to explain such a noticeable difference in adsorption capacities of these dyes by MgO nano-sheets. The used MgO adsorbent can be regenerated completely using heat treatment in a furnace.
中文翻译:

用于从水溶液中吸附阴离子染料的 MgO 纳米片:平衡和动力学研究
摘要 采用FE-SEM、XRD、EDX和N2吸附/解吸等方法合成并表征了厚度为26 nm、网状结构的MgO纳米片。合成的氧化镁纳米片用于去除水溶液中的两种阴离子染料污染物(活性橙和活性黄)。获得了平衡和动力学数据,并且与 Langmuir-Freundlich 和 Redlich-Peterson 等温线以及分形类伪二级动力学模型非常吻合。详细研究了溶液pH和温度对合成吸附剂去除效率的影响。MgO纳米片去除活性橙的最大吸附能力比活性黄高30倍以上。提出了吸附机制的差异来解释MgO纳米片对这些染料的吸附能力的显着差异。使用过的 MgO 吸附剂可以在炉中使用热处理完全再生。
更新日期:2020-12-01
中文翻译:

用于从水溶液中吸附阴离子染料的 MgO 纳米片:平衡和动力学研究
摘要 采用FE-SEM、XRD、EDX和N2吸附/解吸等方法合成并表征了厚度为26 nm、网状结构的MgO纳米片。合成的氧化镁纳米片用于去除水溶液中的两种阴离子染料污染物(活性橙和活性黄)。获得了平衡和动力学数据,并且与 Langmuir-Freundlich 和 Redlich-Peterson 等温线以及分形类伪二级动力学模型非常吻合。详细研究了溶液pH和温度对合成吸附剂去除效率的影响。MgO纳米片去除活性橙的最大吸附能力比活性黄高30倍以上。提出了吸附机制的差异来解释MgO纳米片对这些染料的吸附能力的显着差异。使用过的 MgO 吸附剂可以在炉中使用热处理完全再生。



























 京公网安备 11010802027423号
京公网安备 11010802027423号