Molecular Catalysis ( IF 4.6 ) Pub Date : 2020-10-03 , DOI: 10.1016/j.mcat.2020.111217 Takashi Monna , Ken-ichi Fuhshuku
|
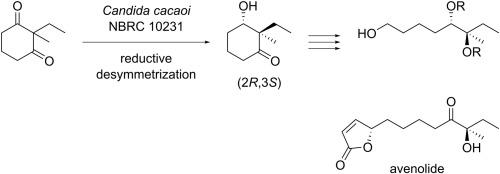
Microorganisms screened from yeast and bacteria were used as biocatalysts to synthesize optically active hydroxyketones with an asymmetric quaternary carbon containing adjacent ethyl and methyl groups based on the microbial reductive desymmetrization of prochiral cyclic 1,3-diketones. Candida cacaoi NBRC 10231 and Pichia farinosa NBRC 10896, two types of yeast species, were found to preferentially reduce only one carbonyl group to give the (2R,3S)-form. In contrast, three types of bacterial species, Morganella morganii NBRC 3168, Providencia rettgeri NBRC 13501, and Escherichia fergusonii NBRC 102419, preferentially produced the (2S,3S)-form with excellent stereoselectivity. Starting from (2R,3S)-hydroxyketone prepared using Candida cacaoi NBRC 10231, which exhibits both high reactivity and stereoselectivity, the characteristic chain structure of avenolide, a type of microbial hormone, was successfully synthesized.
中文翻译:

前手性1,3-二酮的生物催化还原去对称化及其在微生物激素合成中的应用
从酵母和细菌中筛选出的微生物被用作生物催化剂,基于前手性环状1,3-二酮的微生物还原脱对称作用,合成了具有不对称季碳原子的光学活性羟基酮,该季碳原子包含相邻的乙基和甲基。发现两种类型的酵母种类的假丝酵母念珠菌NBRC 10231和粉菌毕赤酵母NBRC 10896仅优先还原一个羰基以提供(2 R,3 S)-形式。相比之下,三种细菌种类,即摩根氏摩根氏菌NBRC 3168,瑞氏普罗维登斯氏菌NBRC 13501和福氏大肠埃希氏菌NBRC 102419,优先产生了(2 S,3 S)-形式具有出色的立体选择性。从使用具有高反应性和立体选择性的假丝酵母念珠菌NBRC 10231制备的(2 R,3 S)-羟基酮开始,成功合成了一种微生物激素-avenolide的特征链结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号