当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Decoding the Papain Inhibitor from Streptomyces mobaraensis as Being Hydroxylated Chymostatin Derivatives: Purification, Structure Analysis, and Putative Biosynthetic Pathway
Journal of Natural Products ( IF 5.1 ) Pub Date : 2020-09-30 , DOI: 10.1021/acs.jnatprod.0c00201 Norbert E Juettner 1, 2 , Jan P Bogen 1 , Tobias A Bauer 1 , Stefan Knapp 3, 4 , Felicitas Pfeifer 2 , Stefan H Huettenhain 1 , Reinhard Meusinger 5 , Andreas Kraemer 3 , Hans-Lothar Fuchsbauer 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2020-09-30 , DOI: 10.1021/acs.jnatprod.0c00201 Norbert E Juettner 1, 2 , Jan P Bogen 1 , Tobias A Bauer 1 , Stefan Knapp 3, 4 , Felicitas Pfeifer 2 , Stefan H Huettenhain 1 , Reinhard Meusinger 5 , Andreas Kraemer 3 , Hans-Lothar Fuchsbauer 1
Affiliation
|
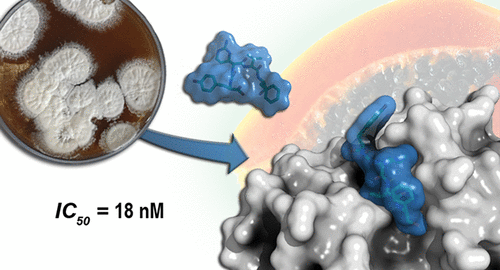
Streptomyces mobaraensis produces the papain inhibitor SPI consisting of a 12 kDa protein and small active compounds (SPIac). Purification of the papain inhibitory compounds resulted in four diverse chymostatin derivatives that were characterized by NMR and MS analysis. Chymostatins are hydrophobic tetrapeptide aldehydes from streptomycetes, e.g., S. lavendulae and S. hygroscopicus, that reverse chymosin-mediated angiotensin activation and inhibit other serine and cysteine proteases. Chymotrypsin and papain were both inhibited by the SPIac compounds in the low nanomolar range. SPIac differs from the characterized chymostatins by the exchange of phenylalanine for tyrosine. The crystal structure of one of these chymostatin variants confirmed its molecular structure and revealed a S-configured hemithioacetal bond with the catalytic Cys25 thiolate as well as close interactions with hydrophobic S1 and S2 subsite amino acids. A model for chymostatin biosynthesis is provided based on the discovery of clustered genes encoding several putative nonribosomal peptide synthetases; among them, there is the unusual CstF enzyme that accommodates two canonical amino acid activation domains as well as three peptide carrier protein domains.
中文翻译:

从 Streptomyces mobaraensis 中解码木瓜蛋白酶抑制剂作为羟基化凝乳酶衍生物:纯化、结构分析和假定的生物合成途径
Streptomyces mobaraensis产生木瓜蛋白酶抑制剂 SPI,由 12 kDa 蛋白质和小活性化合物 (SPI ac ) 组成。木瓜蛋白酶抑制化合物的纯化产生了四种不同的凝乳素衍生物,其特征在于 NMR 和 MS 分析。凝乳酶抑制素是来自链霉菌属的疏水性四肽醛,例如S. lavendulae和S. hygroscopicus,可逆转凝乳酶介导的血管紧张素激活并抑制其他丝氨酸和半胱氨酸蛋白酶。胰凝乳蛋白酶和木瓜蛋白酶均被低纳摩尔范围的 SPI ac化合物抑制。SPI交流通过苯丙氨酸与酪氨酸的交换而不同于特征性的糜蛋白酶。这些糜蛋白酶变体之一的晶体结构证实了其分子结构,并揭示了与催化性 Cys25 硫醇盐的 S 型半硫缩醛键,以及与疏水性 S1 和 S2 亚位点氨基酸的密切相互作用。基于编码几种推定的非核糖体肽合成酶的成簇基因的发现,提供了一种凝乳酶生物合成模型;其中,有一种不寻常的 CstF 酶,可容纳两个标准氨基酸激活域以及三个肽载体蛋白域。
更新日期:2020-10-26
中文翻译:

从 Streptomyces mobaraensis 中解码木瓜蛋白酶抑制剂作为羟基化凝乳酶衍生物:纯化、结构分析和假定的生物合成途径
Streptomyces mobaraensis产生木瓜蛋白酶抑制剂 SPI,由 12 kDa 蛋白质和小活性化合物 (SPI ac ) 组成。木瓜蛋白酶抑制化合物的纯化产生了四种不同的凝乳素衍生物,其特征在于 NMR 和 MS 分析。凝乳酶抑制素是来自链霉菌属的疏水性四肽醛,例如S. lavendulae和S. hygroscopicus,可逆转凝乳酶介导的血管紧张素激活并抑制其他丝氨酸和半胱氨酸蛋白酶。胰凝乳蛋白酶和木瓜蛋白酶均被低纳摩尔范围的 SPI ac化合物抑制。SPI交流通过苯丙氨酸与酪氨酸的交换而不同于特征性的糜蛋白酶。这些糜蛋白酶变体之一的晶体结构证实了其分子结构,并揭示了与催化性 Cys25 硫醇盐的 S 型半硫缩醛键,以及与疏水性 S1 和 S2 亚位点氨基酸的密切相互作用。基于编码几种推定的非核糖体肽合成酶的成簇基因的发现,提供了一种凝乳酶生物合成模型;其中,有一种不寻常的 CstF 酶,可容纳两个标准氨基酸激活域以及三个肽载体蛋白域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号