当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Wide interlayer spacing ammonium vanadate (NH4)0.37V2O5.0.15(H2O) cathode for rechargeable aqueous zinc-ion batteries
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jiec.2020.09.021 Muthusamy Tamilselvan , Thupakula Venkata Madhukar Sreekanth , KisooYoo , Jonghoon Kim
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jiec.2020.09.021 Muthusamy Tamilselvan , Thupakula Venkata Madhukar Sreekanth , KisooYoo , Jonghoon Kim
|
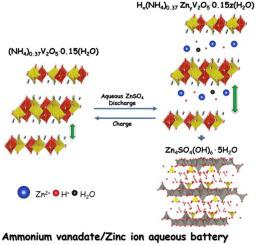
Abstract Recently, aqueous zinc-ion batteries (AZIBs) have been gaining widespread academic interest in the energy-storage field owing to their high energy density, enhanced safety of electrochemical operation, and low cost, as well as the abundance of zinc on earth. The ammonium vanadate group contains compounds that are considered efficient energy-storage materials because they provide two-dimensional (2D) layered structures with large interlayer distances that promote the intercalation of metal ions during electrochemical processes. Here, we report a hydrated form of ammonium vanadate (NH4)0.37V2O5·0.15(H2O) with a highly crystalline rose-like microstructure, which is used as the positive electrode material in AZIBs. The ammonium vanadate cathode delivered an initial capacity of 400 mA h g−1 with 2 M ZnSO4 at the current density of 0.5 Ag−1 in a voltage range from 0.2 V to 1.4 V vs Zn2+/Zn. At a higher current density (10 A g−1), the material retained 84% of the initial discharge capacity after 1000 cycles, while displaying an excellent rate capability. Cyclic voltammetry and ex-situ XRD and XPS are used to study the reaction mechanism of ammonium vanadate cathode in the AZIBs. Unlike other ammonium vanadate’s, (NH4)0.37V2O5·0.15(H2O) undergoes co-intercalation of Zn2+ and H+ along with water molecules. There is no intermediate irreversible crystal phase formation during the discharge/charge cycles due to expandable interlaying distance. This report suggests that (NH4)0.37V2O5·0.15(H2O) can be an alternative highly stable cathode material for use in AZIBs, and it can be promising for the intercalation of the cathode in larger-sized metal-ion storage systems.
中文翻译:

用于可充电水性锌离子电池的宽层间距钒酸铵(NH4)0.37V2O5.0.15(H2O)正极
摘要 近年来,水系锌离子电池(AZIBs)由于其能量密度高、电化学操作安全性高、成本低以及地球上锌含量丰富等优点,在储能领域引起了广泛的学术兴趣。钒酸铵组包含被认为是高效储能材料的化合物,因为它们提供具有大层间距的二维 (2D) 层状结构,可促进电化学过程中金属离子的嵌入。在这里,我们报告了一种水合形式的钒酸铵 (NH4)0.37V2O5·0.15(H2O),具有高度结晶的玫瑰状微观结构,用作 AZIB 的正极材料。钒酸铵正极在电流密度为 0 时提供 400 mA hg-1 的初始容量和 2 M ZnSO4。5 Ag-1 在 0.2 V 至 1.4 V 的电压范围内,相对于 Zn2+/Zn。在较高的电流密度 (10 A g-1) 下,该材料在 1000 次循环后仍保持初始放电容量的 84%,同时显示出优异的倍率性能。循环伏安法和异位 XRD 和 XPS 用于研究 AZIBs 中钒酸铵阴极的反应机理。与其他钒酸铵不同,(NH4)0.37V2O5·0.15(H2O) 经历 Zn2+ 和 H+ 与水分子的共嵌入。由于可扩展的层间距离,在放电/充电循环期间没有中间不可逆晶相形成。该报告表明,(NH4)0.37V2O5·0.15(H2O) 可以作为用于 AZIB 的替代性高稳定性正极材料,并且有望用于将正极嵌入更大尺寸的金属离子存储系统中。
更新日期:2021-01-01
中文翻译:

用于可充电水性锌离子电池的宽层间距钒酸铵(NH4)0.37V2O5.0.15(H2O)正极
摘要 近年来,水系锌离子电池(AZIBs)由于其能量密度高、电化学操作安全性高、成本低以及地球上锌含量丰富等优点,在储能领域引起了广泛的学术兴趣。钒酸铵组包含被认为是高效储能材料的化合物,因为它们提供具有大层间距的二维 (2D) 层状结构,可促进电化学过程中金属离子的嵌入。在这里,我们报告了一种水合形式的钒酸铵 (NH4)0.37V2O5·0.15(H2O),具有高度结晶的玫瑰状微观结构,用作 AZIB 的正极材料。钒酸铵正极在电流密度为 0 时提供 400 mA hg-1 的初始容量和 2 M ZnSO4。5 Ag-1 在 0.2 V 至 1.4 V 的电压范围内,相对于 Zn2+/Zn。在较高的电流密度 (10 A g-1) 下,该材料在 1000 次循环后仍保持初始放电容量的 84%,同时显示出优异的倍率性能。循环伏安法和异位 XRD 和 XPS 用于研究 AZIBs 中钒酸铵阴极的反应机理。与其他钒酸铵不同,(NH4)0.37V2O5·0.15(H2O) 经历 Zn2+ 和 H+ 与水分子的共嵌入。由于可扩展的层间距离,在放电/充电循环期间没有中间不可逆晶相形成。该报告表明,(NH4)0.37V2O5·0.15(H2O) 可以作为用于 AZIB 的替代性高稳定性正极材料,并且有望用于将正极嵌入更大尺寸的金属离子存储系统中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号