当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modular Conjugation of a Potent Anti-HER2 Immunotoxin Using Coassociating Peptides
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-09-30 , DOI: 10.1021/acs.bioconjchem.0c00482 Audrey Stoessel 1 , Nadja Groysbeck 1 , Lucile Guyot 2, 3 , Lina Barret 2 , Yves Nominé 4 , Leonel Nguekeu-Zebaze 1 , Ambre Bender 1 , Laetitia Voilquin 4 , Thomas Lutz 1 , Nikita Pallaoro 1 , Marie Blocat 1 , Celia Deville 4 , Murielle Masson 1 , Guy Zuber 1 , Bruno Chatton 1 , Mariel Donzeau 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-09-30 , DOI: 10.1021/acs.bioconjchem.0c00482 Audrey Stoessel 1 , Nadja Groysbeck 1 , Lucile Guyot 2, 3 , Lina Barret 2 , Yves Nominé 4 , Leonel Nguekeu-Zebaze 1 , Ambre Bender 1 , Laetitia Voilquin 4 , Thomas Lutz 1 , Nikita Pallaoro 1 , Marie Blocat 1 , Celia Deville 4 , Murielle Masson 1 , Guy Zuber 1 , Bruno Chatton 1 , Mariel Donzeau 1
Affiliation
|
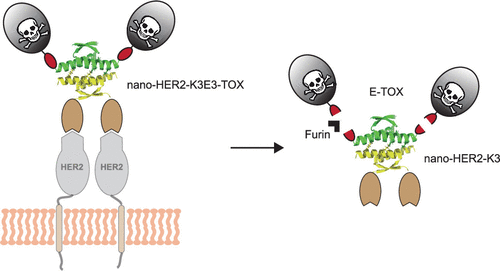
Immunotoxins are emerging candidates for cancer therapeutics. These biomolecules consist of a cell-targeting protein combined to a polypeptide toxin. Associations of both entities can be achieved either chemically by covalent bonds or genetically creating fusion proteins. However, chemical agents can affect the activity and/or stability of the conjugate proteins, and additional purification steps are often required to isolate the final conjugate from unwanted byproducts. As for fusion proteins, they often suffer from low solubility and yield. In this report, we describe a straightforward conjugation process to generate an immunotoxin using coassociating peptides (named K3 and E3), originating from the tetramerization domain of p53. To that end, a nanobody targeting the human epidermal growth factor receptor 2 (nano-HER2) and a protein toxin fragment from Pseudomonas aeruginosa exotoxin A (TOX) were genetically fused to the E3 and K3 peptides. Entities were produced separately in Escherichia coli in soluble forms and at high yields. The nano-HER2 fused to the E3 or K3 helixes (nano-HER2-E3 and nano-HER2-K3) and the coassembled immunotoxins (nano-HER2-K3E3-TOX and nano-HER2-E3K3-TOX) presented binding specificity on HER2-overexpressing cells with relative binding constants in the low nanomolar to picomolar range. Both toxin modules (E3-TOX and K3-TOX) and the combined immunotoxins exhibited similar cytotoxicity levels compared to the toxin alone (TOX). Finally, nano-HER2-K3E3-TOX and nano-HER2-E3K3-TOX evaluated on various breast cancer cells were highly potent and specific to killing HER2-overexpressing breast cancer cells with IC50 values in the picomolar range. Altogether, we demonstrate that this noncovalent conjugation method using two coassembling peptides can be easily implemented for the modular engineering of immunotoxins targeting different types of cancers.
中文翻译:

使用联合肽对强效抗 HER2 免疫毒素进行模块化偶联
免疫毒素是癌症治疗的新兴候选药物。这些生物分子由结合了多肽毒素的细胞靶向蛋白组成。两种实体的结合可以通过共价键以化学方式实现,也可以通过基因产生融合蛋白来实现。然而,化学试剂会影响偶联蛋白的活性和/或稳定性,并且通常需要额外的纯化步骤来将最终偶联物与不需要的副产物分离。至于融合蛋白,它们通常具有低溶解度和低产量的问题。在本报告中,我们描述了一个简单的结合过程,使用源自 p53 的四聚化结构域的联合肽(命名为 K3 和 E3)生成免疫毒素。为此,铜绿假单胞菌外毒素 A (TOX) 在基因上与 E3 和 K3 肽融合。实体在大肠杆菌中以可溶形式和高产率单独产生。与 E3 或 K3 螺旋(nano-HER2-E3 和 nano-HER2-K3)融合的 nano-HER2 和共组装的免疫毒素(nano-HER2-K3E3-TOX 和 nano-HER2-E3K3-TOX)对 HER2 具有结合特异性-在低纳摩尔至皮摩尔范围内的相对结合常数过表达的细胞。与单独的毒素 (TOX) 相比,毒素模块 (E3-TOX 和 K3-TOX) 和组合免疫毒素都表现出相似的细胞毒性水平。最后,对各种乳腺癌细胞进行评估的 nano-HER2-K3E3-TOX 和 nano-HER2-E3K3-TOX 对杀死过表达 HER2 的乳腺癌细胞具有高效和特异性,IC 50皮摩尔范围内的值。总而言之,我们证明了这种使用两种共组装肽的非共价缀合方法可以很容易地用于针对不同类型癌症的免疫毒素的模块化工程。
更新日期:2020-10-21
中文翻译:

使用联合肽对强效抗 HER2 免疫毒素进行模块化偶联
免疫毒素是癌症治疗的新兴候选药物。这些生物分子由结合了多肽毒素的细胞靶向蛋白组成。两种实体的结合可以通过共价键以化学方式实现,也可以通过基因产生融合蛋白来实现。然而,化学试剂会影响偶联蛋白的活性和/或稳定性,并且通常需要额外的纯化步骤来将最终偶联物与不需要的副产物分离。至于融合蛋白,它们通常具有低溶解度和低产量的问题。在本报告中,我们描述了一个简单的结合过程,使用源自 p53 的四聚化结构域的联合肽(命名为 K3 和 E3)生成免疫毒素。为此,铜绿假单胞菌外毒素 A (TOX) 在基因上与 E3 和 K3 肽融合。实体在大肠杆菌中以可溶形式和高产率单独产生。与 E3 或 K3 螺旋(nano-HER2-E3 和 nano-HER2-K3)融合的 nano-HER2 和共组装的免疫毒素(nano-HER2-K3E3-TOX 和 nano-HER2-E3K3-TOX)对 HER2 具有结合特异性-在低纳摩尔至皮摩尔范围内的相对结合常数过表达的细胞。与单独的毒素 (TOX) 相比,毒素模块 (E3-TOX 和 K3-TOX) 和组合免疫毒素都表现出相似的细胞毒性水平。最后,对各种乳腺癌细胞进行评估的 nano-HER2-K3E3-TOX 和 nano-HER2-E3K3-TOX 对杀死过表达 HER2 的乳腺癌细胞具有高效和特异性,IC 50皮摩尔范围内的值。总而言之,我们证明了这种使用两种共组装肽的非共价缀合方法可以很容易地用于针对不同类型癌症的免疫毒素的模块化工程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号