当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A clostripain‐like protease plays a major role in generating the secretome of enterotoxigenic Bacteroides fragilis
Molecular Microbiology ( IF 3.6 ) Pub Date : 2020-09-29 , DOI: 10.1111/mmi.14616 Jessica V Pierce 1 , Justin D Fellows 1 , D Eric Anderson 2 , Harris D Bernstein 1
Molecular Microbiology ( IF 3.6 ) Pub Date : 2020-09-29 , DOI: 10.1111/mmi.14616 Jessica V Pierce 1 , Justin D Fellows 1 , D Eric Anderson 2 , Harris D Bernstein 1
Affiliation
|
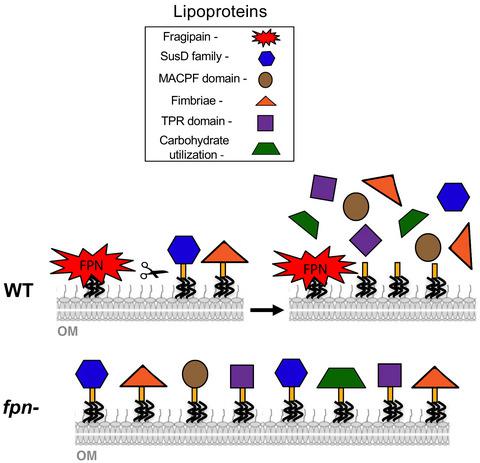
Bacteroides fragilis toxin (BFT) is a protein secreted by enterotoxigenic (ETBF) strains of B. fragilis. BFT is synthesized as a proprotein (proBFT) that is predicted to be a lipoprotein and that is cleaved into two discrete fragments by a clostripain‐like protease called fragipain (Fpn). In this study, we obtained evidence that Fpn cleaves proBFT following its transport across the outer membrane. Remarkably, we also found that the disruption of the fpn gene led to a strong reduction in the level of >100 other proteins, many of which are predicted to be lipoproteins, in the culture medium of an ETBF strain. Experiments performed with purified Fpn provided direct evidence that the protease releases at least some of these proteins from the cell surface. The observation that wild‐type cells outcompeted an fpn‐ strain in co‐cultivation assays also supported the notion that Fpn plays an important role in cell physiology and is not simply dedicated to toxin biogenesis. Finally, we found that purified Fpn altered the adhesive properties of HT29 intestinal epithelial cells. Our results suggest that Fpn is a broad‐spectrum protease that not only catalyzes the protein secretion on a wide scale but that also potentially cleaves host cell proteins during colonization.
中文翻译:

梭菌蛋白酶样蛋白酶在产生产肠毒素的脆弱拟杆菌的分泌组中起主要作用
脆弱拟杆菌毒素 (BFT) 是由脆弱拟杆菌(ETBF) 菌株分泌的一种蛋白质。BFT 被合成为一种前蛋白 (proBFT),预计它是一种脂蛋白,并被称为 fragipain (Fpn) 的梭菌蛋白酶样蛋白酶切割成两个离散的片段。在这项研究中,我们获得了 Fpn 在 proBFT 跨外膜转运后切割它的证据。值得注意的是,我们还发现fpn的中断基因导致ETBF菌株培养基中> 100种其他蛋白质的水平大幅降低,其中许多预计是脂蛋白。用纯化的 Fpn 进行的实验提供了蛋白酶从细胞表面释放至少一些这些蛋白质的直接证据。野生型细胞在共培养试验中胜过 fpn 菌株的观察结果也支持了这样一种观点,即 Fpn 在细胞生理学中起重要作用,而不仅仅致力于毒素的生物合成。最后,我们发现纯化的 Fpn 改变了 HT29 肠上皮细胞的粘附特性。我们的研究结果表明,Fpn 是一种广谱蛋白酶,它不仅可以大规模催化蛋白质分泌,而且还可能在定殖过程中切割宿主细胞蛋白质。
更新日期:2020-09-29
中文翻译:

梭菌蛋白酶样蛋白酶在产生产肠毒素的脆弱拟杆菌的分泌组中起主要作用
脆弱拟杆菌毒素 (BFT) 是由脆弱拟杆菌(ETBF) 菌株分泌的一种蛋白质。BFT 被合成为一种前蛋白 (proBFT),预计它是一种脂蛋白,并被称为 fragipain (Fpn) 的梭菌蛋白酶样蛋白酶切割成两个离散的片段。在这项研究中,我们获得了 Fpn 在 proBFT 跨外膜转运后切割它的证据。值得注意的是,我们还发现fpn的中断基因导致ETBF菌株培养基中> 100种其他蛋白质的水平大幅降低,其中许多预计是脂蛋白。用纯化的 Fpn 进行的实验提供了蛋白酶从细胞表面释放至少一些这些蛋白质的直接证据。野生型细胞在共培养试验中胜过 fpn 菌株的观察结果也支持了这样一种观点,即 Fpn 在细胞生理学中起重要作用,而不仅仅致力于毒素的生物合成。最后,我们发现纯化的 Fpn 改变了 HT29 肠上皮细胞的粘附特性。我们的研究结果表明,Fpn 是一种广谱蛋白酶,它不仅可以大规模催化蛋白质分泌,而且还可能在定殖过程中切割宿主细胞蛋白质。


























 京公网安备 11010802027423号
京公网安备 11010802027423号