当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nonredundant functions of Mycobacterium tuberculosis chaperones promote survival under stress
Molecular Microbiology ( IF 3.6 ) Pub Date : 2020-09-29 , DOI: 10.1111/mmi.14615 Alexa Harnagel 1 , Landys Lopez Quezada 2 , Sae Woong Park 2 , Catherine Baranowski 3 , Karen Kieser 3 , Xiuju Jiang 2 , Julia Roberts 2 , Julien Vaubourgeix 2 , Amy Yang 1 , Brock Nelson 1 , Allison Fay 4 , Eric Rubin 4 , Sabine Ehrt 2 , Carl Nathan 2 , Tania J Lupoli 1, 2
Molecular Microbiology ( IF 3.6 ) Pub Date : 2020-09-29 , DOI: 10.1111/mmi.14615 Alexa Harnagel 1 , Landys Lopez Quezada 2 , Sae Woong Park 2 , Catherine Baranowski 3 , Karen Kieser 3 , Xiuju Jiang 2 , Julia Roberts 2 , Julien Vaubourgeix 2 , Amy Yang 1 , Brock Nelson 1 , Allison Fay 4 , Eric Rubin 4 , Sabine Ehrt 2 , Carl Nathan 2 , Tania J Lupoli 1, 2
Affiliation
|
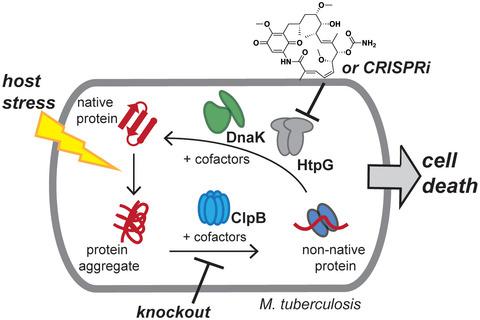
Bacterial chaperones ClpB and DnaK, homologs of the respective eukaryotic heat shock proteins Hsp104 and Hsp70, are essential in the reactivation of toxic protein aggregates that occur during translation or periods of stress. In the pathogen Mycobacterium tuberculosis (Mtb), the protective effect of chaperones extends to survival in the presence of host stresses, such as protein‐damaging oxidants. However, we lack a full understanding of the interplay of Hsps and other stress response genes in mycobacteria. Here, we employ genome‐wide transposon mutagenesis to identify the genes that support clpB function in Mtb. In addition to validating the role of ClpB in Mtb's response to oxidants, we show that HtpG, a homolog of Hsp90, plays a distinct role from ClpB in the proteotoxic stress response. While loss of neither clpB nor htpG is lethal to the cell, loss of both through genetic depletion or small molecule inhibition impairs recovery after exposure to host‐like stresses, especially reactive nitrogen species. Moreover, defects in cells lacking clpB can be complemented by overexpression of other chaperones, demonstrating that Mtb's stress response network depends upon finely tuned chaperone expression levels. These results suggest that inhibition of multiple chaperones could work in concert with host immunity to disable Mtb.
中文翻译:

结核分枝杆菌伴侣的非冗余功能促进应激下的生存
细菌伴侣 ClpB 和 DnaK 是各自真核热休克蛋白 Hsp104 和 Hsp70 的同源物,在翻译或应激期间发生的有毒蛋白质聚集体的再激活中是必不可少的。在病原体结核分枝杆菌(Mtb) 中,分子伴侣的保护作用延伸到存在宿主压力(如蛋白质破坏性氧化剂)下的生存。然而,我们对分枝杆菌中 Hsps 和其他应激反应基因的相互作用缺乏充分的了解。在这里,我们采用全基因组转座子诱变来鉴定支持clpB的基因在 Mtb 中的功能。除了验证 ClpB 在 Mtb 对氧化剂的反应中的作用外,我们还表明 HtpG(Hsp90 的同源物)在蛋白毒性应激反应中与 ClpB 具有不同的作用。虽然clpB和htpG的丧失对细胞都不是致命的,但通过基因耗竭或小分子抑制而丧失两者都会损害暴露于宿主样应激,尤其是活性氮物种后的恢复。此外,缺乏clpB的细胞中的缺陷可以通过其他伴侣的过度表达来补充,这表明 Mtb 的应激反应网络取决于微调的伴侣表达水平。这些结果表明,多种伴侣的抑制可以与宿主免疫协同工作以禁用 Mtb。
更新日期:2020-09-29
中文翻译:

结核分枝杆菌伴侣的非冗余功能促进应激下的生存
细菌伴侣 ClpB 和 DnaK 是各自真核热休克蛋白 Hsp104 和 Hsp70 的同源物,在翻译或应激期间发生的有毒蛋白质聚集体的再激活中是必不可少的。在病原体结核分枝杆菌(Mtb) 中,分子伴侣的保护作用延伸到存在宿主压力(如蛋白质破坏性氧化剂)下的生存。然而,我们对分枝杆菌中 Hsps 和其他应激反应基因的相互作用缺乏充分的了解。在这里,我们采用全基因组转座子诱变来鉴定支持clpB的基因在 Mtb 中的功能。除了验证 ClpB 在 Mtb 对氧化剂的反应中的作用外,我们还表明 HtpG(Hsp90 的同源物)在蛋白毒性应激反应中与 ClpB 具有不同的作用。虽然clpB和htpG的丧失对细胞都不是致命的,但通过基因耗竭或小分子抑制而丧失两者都会损害暴露于宿主样应激,尤其是活性氮物种后的恢复。此外,缺乏clpB的细胞中的缺陷可以通过其他伴侣的过度表达来补充,这表明 Mtb 的应激反应网络取决于微调的伴侣表达水平。这些结果表明,多种伴侣的抑制可以与宿主免疫协同工作以禁用 Mtb。


























 京公网安备 11010802027423号
京公网安备 11010802027423号