Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-09-30 , DOI: 10.1016/j.jmb.2020.09.017 Yuki Kawasaki , Hirotaka Ariyama , Hajime Motomura , Daisuke Fujinami , Daisuke Noshiro , Toshio Ando , Daisuke Kohda
|
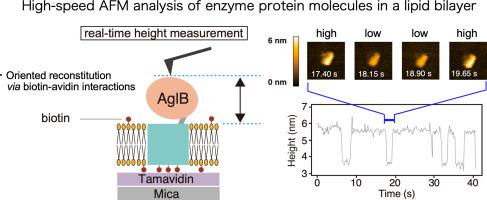
Oligosaccharyltransferase (OST) is a membrane-bound enzyme that catalyzes the transfer of oligosaccharide chains from lipid-linked oligosaccharides (LLO) to asparagine residues in polypeptide chains. Using high-speed atomic force microscopy (AFM), we investigated the dynamic properties of OST molecules embedded in biomembranes. An archaeal single-subunit OST protein was immobilized on a mica support via biotin–avidin interactions and reconstituted in a lipid bilayer. The distance between the top of the protein molecule and the upper surface of the lipid bilayer was monitored in real-time. The height of the extramembranous part exhibited a two-step variation with a difference of 1.8 nm. The high and low states are designated as state 1 and state 2, respectively. The transition processes between the two states fit well to single exponential functions, suggesting that the observed dynamic exchange is an intrinsic property of the archaeal OST protein. The two sets of cross peaks in the NMR spectra of the protein supported the conformational changes between the two states in detergent-solubilized conditions. Considering the height values measured in the AFM measurements, state 1 is closer to the crystal structure, and state 2 has a more compact form. Subsequent AFM experiments indicated that the binding of the sugar donor LLO decreased the structural fluctuation and shifted the equilibrium almost completely to state 1. This dynamic behavior is likely necessary for efficient catalytic turnover. Presumably, state 2 facilitates the immediate release of the bulky glycosylated polypeptide product, thus allowing OST to quickly prepare for the next catalytic cycle.
中文翻译:

高速原子力显微镜实时观察的膜嵌入寡糖基转移酶的二态交换动力学
寡糖基转移酶(OST)是一种膜结合酶,可催化寡糖链从脂质连接的寡糖(LLO)转移至多肽链中的天冬酰胺残基。使用高速原子力显微镜(AFM),我们研究了嵌入生物膜中的OST分子的动态特性。将古细菌单亚基OST蛋白通过固定在云母载体上生物素-亲和素相互作用,并在脂质双层中重构。实时监测蛋白质分子顶部和脂质双层上表面之间的距离。膜外部分的高度表现出两步变化,相差1.8 nm。高状态和低状态分别指定为状态1和状态2。两种状态之间的过渡过程非常适合单个指数函数,这表明观察到的动态交换是古细菌OST蛋白的固有特性。蛋白质的NMR光谱中的两组交叉峰支持在去污剂增溶条件下两种状态之间的构象变化。考虑到在AFM测量中测得的高度值,状态1更接近晶体结构,状态2具有更紧凑的形式。随后的AFM实验表明,糖供体LLO的结合减少了结构波动并使平衡几乎完全转变为状态1。这种动态行为可能是有效催化转换所必需的。据推测,状态2促进了松散的糖基化多肽产物的立即释放,从而使OST快速准备了下一个催化循环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号