当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental investigation and thermodynamic modeling of equilibrium extraction of gold(III) from hydrochloric acid in 1-butyl-3-methylimidazolium hexafluorophosphate
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.fluid.2020.112839 Samira Farzam , Farzaneh Feyzi
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.fluid.2020.112839 Samira Farzam , Farzaneh Feyzi
|
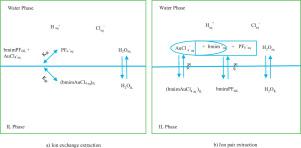
Abstract Liquid-liquid equilibrium (LLE) characterization of ionic liquid (IL) systems is an essential method to examine ILs as potential alternative solvents for traditional extraction and separation which also affirms the significance of modeling IL containing mixtures. In the present study, extraction of gold(III) from an acidic medium was performed using 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim][PF6]) at 25°C for which the reaction mechanism was investigated. In order to model the above mentioned system, the implicit version of mean spherical approximation model (MSA) was added to Statistical Associating Fluid Theory Equation of State (SAFT-HR EoS) developed by Huang and Radosz. Non-idealities of both aqueous and organic phases were taken into account. Water was assumed to have two association sites and each ion was considered as a spherical species exerting repulsive, coulombic, and dispersive interactions. Two approaches were considered to model [bmim][PF6] as an extractant; the ion-based and the salt-based. A set of EoS parameters for each approach was obtained by fitting to reliable density data of [bmim][PF6] from literature with fitting error of less than 0.31% on average. The theory is found to accurately model the experimental equilibrium concentration of gold(III) with the average error of 9.62%. The predictive capability of the model was studied, as well. The results show that the model is a powerful tool for modeling the extraction system containing water, ions and IL.
中文翻译:

盐酸中1-丁基-3-甲基咪唑鎓六氟磷酸酯平衡萃取金(III)的实验研究和热力学模拟
摘要 离子液体 (IL) 系统的液-液平衡 (LLE) 表征是检查离子液体作为传统萃取和分离的潜在替代溶剂的重要方法,这也肯定了对含有离子液体的混合物进行建模的重要性。在本研究中,使用 1-丁基-3-甲基咪唑鎓六氟磷酸盐 ([bmim][PF6]) 在 25°C 下从酸性介质中提取金 (III),并研究了反应机理。为了对上述系统进行建模,在 Huang 和 Radosz 开发的统计关联流体理论状态方程 (SAFT-HR EoS) 中添加了平均球面近似模型 (MSA) 的隐式版本。考虑了水相和有机相的非理想性。假设水有两个缔合位点,每个离子都被认为是一种球形物质,会产生排斥、库仑和色散相互作用。考虑了两种方法将 [bmim][PF6] 建模为萃取剂;离子型和盐型。每种方法的一组 EoS 参数是通过拟合文献中可靠的 [bmim][PF6] 密度数据获得的,拟合误差平均小于 0.31%。发现该理论可以准确地模拟金 (III) 的实验平衡浓度,平均误差为 9.62%。还研究了模型的预测能力。结果表明,该模型是对含有水、离子和离子液体的萃取系统进行建模的有力工具。考虑了两种方法将 [bmim][PF6] 建模为萃取剂;离子型和盐型。每种方法的一组 EoS 参数是通过拟合文献中可靠的 [bmim][PF6] 密度数据获得的,拟合误差平均小于 0.31%。发现该理论可以准确地模拟金 (III) 的实验平衡浓度,平均误差为 9.62%。还研究了模型的预测能力。结果表明,该模型是对含有水、离子和离子液体的萃取系统进行建模的有力工具。考虑了两种方法将 [bmim][PF6] 建模为萃取剂;离子型和盐型。每种方法的一组 EoS 参数是通过拟合文献中可靠的 [bmim][PF6] 密度数据获得的,拟合误差平均小于 0.31%。发现该理论可以准确地模拟金 (III) 的实验平衡浓度,平均误差为 9.62%。还研究了模型的预测能力。结果表明,该模型是对含有水、离子和离子液体的萃取系统进行建模的有力工具。发现该理论可以准确地模拟金 (III) 的实验平衡浓度,平均误差为 9.62%。还研究了模型的预测能力。结果表明,该模型是对含有水、离子和离子液体的萃取系统进行建模的有力工具。发现该理论可以准确地模拟金 (III) 的实验平衡浓度,平均误差为 9.62%。还研究了模型的预测能力。结果表明,该模型是对含有水、离子和离子液体的萃取系统进行建模的有力工具。
更新日期:2021-01-01
中文翻译:

盐酸中1-丁基-3-甲基咪唑鎓六氟磷酸酯平衡萃取金(III)的实验研究和热力学模拟
摘要 离子液体 (IL) 系统的液-液平衡 (LLE) 表征是检查离子液体作为传统萃取和分离的潜在替代溶剂的重要方法,这也肯定了对含有离子液体的混合物进行建模的重要性。在本研究中,使用 1-丁基-3-甲基咪唑鎓六氟磷酸盐 ([bmim][PF6]) 在 25°C 下从酸性介质中提取金 (III),并研究了反应机理。为了对上述系统进行建模,在 Huang 和 Radosz 开发的统计关联流体理论状态方程 (SAFT-HR EoS) 中添加了平均球面近似模型 (MSA) 的隐式版本。考虑了水相和有机相的非理想性。假设水有两个缔合位点,每个离子都被认为是一种球形物质,会产生排斥、库仑和色散相互作用。考虑了两种方法将 [bmim][PF6] 建模为萃取剂;离子型和盐型。每种方法的一组 EoS 参数是通过拟合文献中可靠的 [bmim][PF6] 密度数据获得的,拟合误差平均小于 0.31%。发现该理论可以准确地模拟金 (III) 的实验平衡浓度,平均误差为 9.62%。还研究了模型的预测能力。结果表明,该模型是对含有水、离子和离子液体的萃取系统进行建模的有力工具。考虑了两种方法将 [bmim][PF6] 建模为萃取剂;离子型和盐型。每种方法的一组 EoS 参数是通过拟合文献中可靠的 [bmim][PF6] 密度数据获得的,拟合误差平均小于 0.31%。发现该理论可以准确地模拟金 (III) 的实验平衡浓度,平均误差为 9.62%。还研究了模型的预测能力。结果表明,该模型是对含有水、离子和离子液体的萃取系统进行建模的有力工具。考虑了两种方法将 [bmim][PF6] 建模为萃取剂;离子型和盐型。每种方法的一组 EoS 参数是通过拟合文献中可靠的 [bmim][PF6] 密度数据获得的,拟合误差平均小于 0.31%。发现该理论可以准确地模拟金 (III) 的实验平衡浓度,平均误差为 9.62%。还研究了模型的预测能力。结果表明,该模型是对含有水、离子和离子液体的萃取系统进行建模的有力工具。发现该理论可以准确地模拟金 (III) 的实验平衡浓度,平均误差为 9.62%。还研究了模型的预测能力。结果表明,该模型是对含有水、离子和离子液体的萃取系统进行建模的有力工具。发现该理论可以准确地模拟金 (III) 的实验平衡浓度,平均误差为 9.62%。还研究了模型的预测能力。结果表明,该模型是对含有水、离子和离子液体的萃取系统进行建模的有力工具。



























 京公网安备 11010802027423号
京公网安备 11010802027423号