当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermodynamic Models for Determination of Solid–Liquid Equilibrium of the Sarafloxacin Hydrochloride in Pure and Binary Organic Solvents from (278.15 to 333.15) K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-09-29 , DOI: 10.1021/acs.jced.0c00496 Meng Cheng 1 , Xin Zhao 1 , Aixiang Hao 2 , Wenhao Jiao 1 , Wenge Yang 1 , Yonghong Hu 3
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-09-29 , DOI: 10.1021/acs.jced.0c00496 Meng Cheng 1 , Xin Zhao 1 , Aixiang Hao 2 , Wenhao Jiao 1 , Wenge Yang 1 , Yonghong Hu 3
Affiliation
|
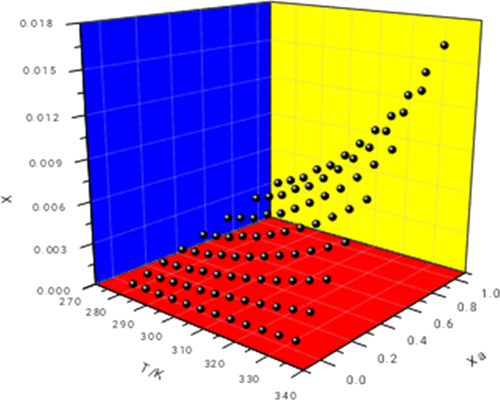
In this study, the gravimetric method was taken to measure the solubility of the sarafloxacin hydrochloride under atmospheric pressure in methanol, isopropanol, ethanol, 1-butanol, acetonitrile, n-hexane, ethyl acetate, and N,N-dimethylformamide as well as in the (ethyl acetate + acetonitrile), (methanol + n-hexane), and (ethyl acetate + n-hexane) mixtures from 278.15 to 333.15 K. The solubility of sarafloxacin hydrochloride increased with increasing temperature in all the solvents. The solid–liquid solubility equilibrium data in the pure and mixed solvents were in apparent agreement with the solubility predicted by the modified Apelblat model, Buchowski–Ksiazaczak λh model, Redlich–Kister (CNIBS/R-K) model, and Jouyban–Acree model. However, the Redlich–Kister (CNIBS/R-K) model best simulated the experimental solubility results.
中文翻译:

测定(278.15至333.15)K的纯和二元有机溶剂中盐酸沙拉沙星固液平衡的热力学模型
在这项研究中,被带到重量分析法来测量下甲醇,异丙醇,乙醇,1-丁醇,乙腈,大气压力的盐酸沙拉沙星的溶解度Ñ己烷,乙酸乙酯,和Ñ,Ñ如二甲基甲酰胺以及在的(乙酸乙酯+乙腈),(甲醇+ ñ己烷),和(乙酸乙酯+ ñ己烷)的混合物从278.15到333.15 K.盐酸沙拉沙星的溶解度,在所有的溶剂增加的温度上升。在纯的和混合的溶剂固液溶解度平衡数据与由所述改性Apelblat模型,Buchowski-Ksiazaczakλ预测溶解度明显协议ħ模型,Redlich–Kister(CNIBS / RK)模型和Jouyban–Acree模型。但是,Redlich–Kister(CNIBS / RK)模型可以最好地模拟实验溶解度结果。
更新日期:2020-10-08
中文翻译:

测定(278.15至333.15)K的纯和二元有机溶剂中盐酸沙拉沙星固液平衡的热力学模型
在这项研究中,被带到重量分析法来测量下甲醇,异丙醇,乙醇,1-丁醇,乙腈,大气压力的盐酸沙拉沙星的溶解度Ñ己烷,乙酸乙酯,和Ñ,Ñ如二甲基甲酰胺以及在的(乙酸乙酯+乙腈),(甲醇+ ñ己烷),和(乙酸乙酯+ ñ己烷)的混合物从278.15到333.15 K.盐酸沙拉沙星的溶解度,在所有的溶剂增加的温度上升。在纯的和混合的溶剂固液溶解度平衡数据与由所述改性Apelblat模型,Buchowski-Ksiazaczakλ预测溶解度明显协议ħ模型,Redlich–Kister(CNIBS / RK)模型和Jouyban–Acree模型。但是,Redlich–Kister(CNIBS / RK)模型可以最好地模拟实验溶解度结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号