当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tuning the Thermal Stability and Photoisomerization of Azoheteroarenes through Macrocycle Strain**
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-09-29 , DOI: 10.1002/chem.202003926 Sergi Vela 1 , Alan Scheidegger 1 , Raimon Fabregat 1 , Clémence Corminboeuf 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-09-29 , DOI: 10.1002/chem.202003926 Sergi Vela 1 , Alan Scheidegger 1 , Raimon Fabregat 1 , Clémence Corminboeuf 1
Affiliation
|
Azobenzene and its derivatives are one of the most widespread molecular scaffolds used in a range of modern applications, as well as in fundamental research. After photoexcitation, azo‐based photoswitches revert back to the most stable isomer on a timescale ( ) that determines the range of potential applications. Attempts to bring
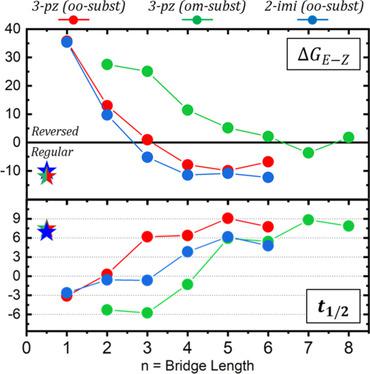
) that determines the range of potential applications. Attempts to bring  to extreme values prompted the development of azobenzene and azoheteroarene derivatives that either rebalance the E‐ and Z‐isomer stabilities, or exploit unconventional thermal isomerization mechanisms. In the former case, one successful strategy has been the creation of macrocycle strain, which tends to impact the E/Z stability asymmetrically, and thus significantly modify
to extreme values prompted the development of azobenzene and azoheteroarene derivatives that either rebalance the E‐ and Z‐isomer stabilities, or exploit unconventional thermal isomerization mechanisms. In the former case, one successful strategy has been the creation of macrocycle strain, which tends to impact the E/Z stability asymmetrically, and thus significantly modify . On the bright side, bridged derivatives have shown an improved optical switching owing to the higher quantum yields and absence of degradation. However, in most (if not all) cases, bridged derivatives display a reversed thermal stability (more stable Z‐isomer), and smaller
. On the bright side, bridged derivatives have shown an improved optical switching owing to the higher quantum yields and absence of degradation. However, in most (if not all) cases, bridged derivatives display a reversed thermal stability (more stable Z‐isomer), and smaller  than the acyclic counterparts, which restricts their potential interest to applications requiring a fast forward and backwards switch. In this paper, the impact of alkyl bridges on the thermal stability of phenyl‐azoheteroarenes is investigated by using computational methods, and it is revealed that it is indeed possible to combine such improved photoswitching characteristics while preserving the regular thermal stability (more stable E‐isomer), and increased
than the acyclic counterparts, which restricts their potential interest to applications requiring a fast forward and backwards switch. In this paper, the impact of alkyl bridges on the thermal stability of phenyl‐azoheteroarenes is investigated by using computational methods, and it is revealed that it is indeed possible to combine such improved photoswitching characteristics while preserving the regular thermal stability (more stable E‐isomer), and increased  values under the appropriate connectivity and bridge length.
values under the appropriate connectivity and bridge length.
中文翻译:

通过大环应变调节偶氮杂芳烃的热稳定性和光异构化**
偶氮苯及其衍生物是在一系列现代应用以及基础研究中使用最广泛的分子支架之一。光激发后,偶氮基光开关在 决定潜在应用范围的时间尺度 ( ) 上恢复到最稳定的异构体。对极端值的尝试促进了
决定潜在应用范围的时间尺度 ( ) 上恢复到最稳定的异构体。对极端值的尝试促进了 偶氮苯和偶氮杂芳烃衍生物的发展,它们要么重新平衡E-和Z-异构体的稳定性,要么利用非常规的热异构化机制。在前一种情况下,一个成功的策略是创建大环应变,这往往会不对称地影响E / Z稳定性,从而显着修改
偶氮苯和偶氮杂芳烃衍生物的发展,它们要么重新平衡E-和Z-异构体的稳定性,要么利用非常规的热异构化机制。在前一种情况下,一个成功的策略是创建大环应变,这往往会不对称地影响E / Z稳定性,从而显着修改 。从好的方面来说,桥联衍生物由于更高的量子产率和不存在退化而显示出改进的光学开关。然而,在大多数(如果不是全部)情况下,桥联衍生物表现出相反的热稳定性(更稳定的Z异构体),并且
。从好的方面来说,桥联衍生物由于更高的量子产率和不存在退化而显示出改进的光学开关。然而,在大多数(如果不是全部)情况下,桥联衍生物表现出相反的热稳定性(更稳定的Z异构体),并且 比无环对应物小,这限制了它们对需要快速向前和向后切换的应用的潜在兴趣。本文通过计算方法研究了烷基桥对苯基偶氮杂芳烃热稳定性的影响,结果表明确实可以将这种改进的光开关特性结合起来,同时保持常规的热稳定性(更稳定的E-异构体),并
比无环对应物小,这限制了它们对需要快速向前和向后切换的应用的潜在兴趣。本文通过计算方法研究了烷基桥对苯基偶氮杂芳烃热稳定性的影响,结果表明确实可以将这种改进的光开关特性结合起来,同时保持常规的热稳定性(更稳定的E-异构体),并 在适当的连接性和桥长度下增加值。
在适当的连接性和桥长度下增加值。
更新日期:2020-09-29
 ) that determines the range of potential applications. Attempts to bring
) that determines the range of potential applications. Attempts to bring  to extreme values prompted the development of azobenzene and azoheteroarene derivatives that either rebalance the E‐ and Z‐isomer stabilities, or exploit unconventional thermal isomerization mechanisms. In the former case, one successful strategy has been the creation of macrocycle strain, which tends to impact the E/Z stability asymmetrically, and thus significantly modify
to extreme values prompted the development of azobenzene and azoheteroarene derivatives that either rebalance the E‐ and Z‐isomer stabilities, or exploit unconventional thermal isomerization mechanisms. In the former case, one successful strategy has been the creation of macrocycle strain, which tends to impact the E/Z stability asymmetrically, and thus significantly modify . On the bright side, bridged derivatives have shown an improved optical switching owing to the higher quantum yields and absence of degradation. However, in most (if not all) cases, bridged derivatives display a reversed thermal stability (more stable Z‐isomer), and smaller
. On the bright side, bridged derivatives have shown an improved optical switching owing to the higher quantum yields and absence of degradation. However, in most (if not all) cases, bridged derivatives display a reversed thermal stability (more stable Z‐isomer), and smaller  than the acyclic counterparts, which restricts their potential interest to applications requiring a fast forward and backwards switch. In this paper, the impact of alkyl bridges on the thermal stability of phenyl‐azoheteroarenes is investigated by using computational methods, and it is revealed that it is indeed possible to combine such improved photoswitching characteristics while preserving the regular thermal stability (more stable E‐isomer), and increased
than the acyclic counterparts, which restricts their potential interest to applications requiring a fast forward and backwards switch. In this paper, the impact of alkyl bridges on the thermal stability of phenyl‐azoheteroarenes is investigated by using computational methods, and it is revealed that it is indeed possible to combine such improved photoswitching characteristics while preserving the regular thermal stability (more stable E‐isomer), and increased  values under the appropriate connectivity and bridge length.
values under the appropriate connectivity and bridge length.
中文翻译:

通过大环应变调节偶氮杂芳烃的热稳定性和光异构化**
偶氮苯及其衍生物是在一系列现代应用以及基础研究中使用最广泛的分子支架之一。光激发后,偶氮基光开关在
 决定潜在应用范围的时间尺度 ( ) 上恢复到最稳定的异构体。对极端值的尝试促进了
决定潜在应用范围的时间尺度 ( ) 上恢复到最稳定的异构体。对极端值的尝试促进了 偶氮苯和偶氮杂芳烃衍生物的发展,它们要么重新平衡E-和Z-异构体的稳定性,要么利用非常规的热异构化机制。在前一种情况下,一个成功的策略是创建大环应变,这往往会不对称地影响E / Z稳定性,从而显着修改
偶氮苯和偶氮杂芳烃衍生物的发展,它们要么重新平衡E-和Z-异构体的稳定性,要么利用非常规的热异构化机制。在前一种情况下,一个成功的策略是创建大环应变,这往往会不对称地影响E / Z稳定性,从而显着修改 。从好的方面来说,桥联衍生物由于更高的量子产率和不存在退化而显示出改进的光学开关。然而,在大多数(如果不是全部)情况下,桥联衍生物表现出相反的热稳定性(更稳定的Z异构体),并且
。从好的方面来说,桥联衍生物由于更高的量子产率和不存在退化而显示出改进的光学开关。然而,在大多数(如果不是全部)情况下,桥联衍生物表现出相反的热稳定性(更稳定的Z异构体),并且 比无环对应物小,这限制了它们对需要快速向前和向后切换的应用的潜在兴趣。本文通过计算方法研究了烷基桥对苯基偶氮杂芳烃热稳定性的影响,结果表明确实可以将这种改进的光开关特性结合起来,同时保持常规的热稳定性(更稳定的E-异构体),并
比无环对应物小,这限制了它们对需要快速向前和向后切换的应用的潜在兴趣。本文通过计算方法研究了烷基桥对苯基偶氮杂芳烃热稳定性的影响,结果表明确实可以将这种改进的光开关特性结合起来,同时保持常规的热稳定性(更稳定的E-异构体),并 在适当的连接性和桥长度下增加值。
在适当的连接性和桥长度下增加值。


























 京公网安备 11010802027423号
京公网安备 11010802027423号