Green Synthesis and Catalysis Pub Date : 2020-07-27 , DOI: 10.1016/j.gresc.2020.06.001 Qinyue Deng , Qingshu Zheng , Bin Zuo , Tao Tu
|
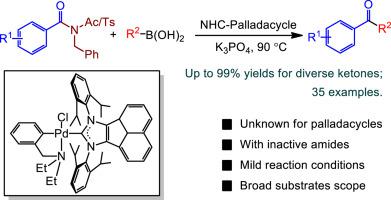
Robust NHC-palladacycles (NHC = N-heterocyclic carbene) were synthesized and exhibited high catalytic activity towards Suzuki−Miyaura cross-coupling reactions between inactive amides with N-acetyl/benzyl substituents and aryl boronic acids, producing diverse ketones in good to excellent yields. This unprecedented and practical palladacycles-catalyzed Suzuki−Miyaura cross-coupling of amides with boronic acids via selective C-N bond activation was attributed to the strong σ-donor and weak π-acceptor properties of acenaphthoimidazolylidene, which may highlight their potential in other challenging coupling transformations involving inactive amides.
中文翻译:

通过CN活化,鲁棒的NHC-Palladacycles催化酰胺的Suzuki-Miyaura交叉偶联
合成了健壮的NHC-四环五环(NHC = N-杂环卡宾),并表现出对具有N-乙酰基/苄基取代基的非活性酰胺与芳基硼酸之间的Suzuki-Miyaura交叉偶联反应具有高催化活性,从而产生了多种酮体,收率好至极佳。通过选择性CN键活化,这种空前的,实用的palladacycles催化酰胺与硼酸的Suzuki-Miyaura交叉偶联,归因于啶咪唑基亚甲基的强σ-供体和弱π-受体性质,这可能突出了它们在其他挑战性偶合转化中的潜力涉及非活性酰胺。


























 京公网安备 11010802027423号
京公网安备 11010802027423号