当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Corrosion inhibition of carbon steel in hydrochloric acid solution using newly synthesized urea-based cationic fluorosurfactants: experimental and computational investigations
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020-09-28 , DOI: 10.1039/d0nj04004e Hany M. Abd El-Lateef 1, 2, 3, 4, 5 , K. Shalabi 6, 7, 8, 9, 10 , Ahmed H. Tantawy 6, 7, 10, 11, 12
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020-09-28 , DOI: 10.1039/d0nj04004e Hany M. Abd El-Lateef 1, 2, 3, 4, 5 , K. Shalabi 6, 7, 8, 9, 10 , Ahmed H. Tantawy 6, 7, 10, 11, 12
Affiliation
|
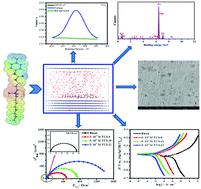
The facile synthesis approach, surface-active properties, and anti-corrosive efficiency of innovative urea-based cationic fluorinated surfactants (FUS) of variable alkyl chain length were investigated. The effect of as-prepared FUS inhibitors on carbon steel corrosion in 15% HCl solution at 20–50 °C was examined using weight loss and various electrochemical methods (open circuit potential (OCP) vs. time, potentiodynamic polarization (PDA) and electrochemical impedance spectroscopy (EIS) methods). The corrosion protection capacities at an optimum concentration (0.5 mM) are 93.2% (FUS-8), 96.3% (FUS-10) and 97.6% (FUS-12) by PDA, comparable to the findings achieved by EIS. PDA measurements indicated that the studied FUS act as inhibitors of mixed-type and chemical adsorption on a carbon steel interface following the Langmuir isotherm model. The thermodynamic and kinetic indices of the corrosion and adsorption processes were estimated and interpreted. Surface examinations by X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy (FTIR), field emission scanning electron microscopy and energy dispersive X-ray analysis (FESEM/EDX) revealed the formation of a protecting layer at the carbon steel/HCl interface. Quantum chemical indices (DFT based) delivered more understanding of the inhibition mechanism. Molecular dynamics (MD) simulations were accomplished to explore the configurational adsorption performance of the studied fluorosurfactants on the iron(110) interface. It is supposed that these findings are of some significance for the reasonable design of effective inhibitors for acidic corrosion.
中文翻译:

新合成的脲基阳离子含氟表面活性剂在盐酸溶液中抑制碳钢腐蚀的实验和计算研究
研究了烷基链长可变的新型脲基阳离子氟化表面活性剂(FUS)的简便合成方法,表面活性和抗腐蚀性能。使用失重法和各种电化学方法(开路电势(OCP)随时间变化,电势极化(PDA)和电化学方法),研究了制备的FUS抑制剂对20%至50°C的15%HCl溶液中碳钢腐蚀的影响。阻抗谱(EIS)方法)。最佳浓度(0.5 mM)时的腐蚀防护能力为93.2%(FUS-8),96.3%(FUS-10)和97.6%(FUS-12),与EIS的发现相当。PDA测量表明,按照Langmuir等温模型,所研究的FUS在碳钢界面上起混合型和化学吸附抑制剂的作用。估算并解释了腐蚀和吸附过程的热力学和动力学指数。通过X射线光电子能谱(XPS),傅里叶变换红外光谱(FTIR),场发射扫描电子显微镜和能量色散X射线分析(FESEM / EDX)进行的表面检查表明,碳钢/ HCl界面。量子化学指数(基于DFT)使人们对抑制机理有了更多的了解。完成了分子动力学(MD)模拟,以探索所研究的含氟表面活性剂在铁(110)界面上的构型吸附性能。可以认为这些发现对于合理设计有效的酸性腐蚀抑制剂具有重要意义。
更新日期:2020-10-11
中文翻译:

新合成的脲基阳离子含氟表面活性剂在盐酸溶液中抑制碳钢腐蚀的实验和计算研究
研究了烷基链长可变的新型脲基阳离子氟化表面活性剂(FUS)的简便合成方法,表面活性和抗腐蚀性能。使用失重法和各种电化学方法(开路电势(OCP)随时间变化,电势极化(PDA)和电化学方法),研究了制备的FUS抑制剂对20%至50°C的15%HCl溶液中碳钢腐蚀的影响。阻抗谱(EIS)方法)。最佳浓度(0.5 mM)时的腐蚀防护能力为93.2%(FUS-8),96.3%(FUS-10)和97.6%(FUS-12),与EIS的发现相当。PDA测量表明,按照Langmuir等温模型,所研究的FUS在碳钢界面上起混合型和化学吸附抑制剂的作用。估算并解释了腐蚀和吸附过程的热力学和动力学指数。通过X射线光电子能谱(XPS),傅里叶变换红外光谱(FTIR),场发射扫描电子显微镜和能量色散X射线分析(FESEM / EDX)进行的表面检查表明,碳钢/ HCl界面。量子化学指数(基于DFT)使人们对抑制机理有了更多的了解。完成了分子动力学(MD)模拟,以探索所研究的含氟表面活性剂在铁(110)界面上的构型吸附性能。可以认为这些发现对于合理设计有效的酸性腐蚀抑制剂具有重要意义。


























 京公网安备 11010802027423号
京公网安备 11010802027423号