当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Near UV and Visible Light Induce Iron-Dependent Photodegradation Reactions in Pharmaceutical Buffers: Mechanistic and Product Studies
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-09-28 , DOI: 10.1021/acs.molpharmaceut.0c00639 Natalia Subelzu 1 , Christian Schöneich 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-09-28 , DOI: 10.1021/acs.molpharmaceut.0c00639 Natalia Subelzu 1 , Christian Schöneich 1
Affiliation
|
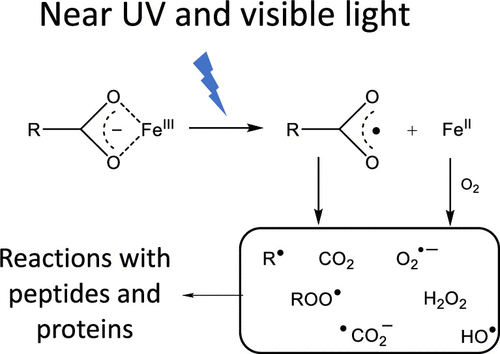
Near UV (λ = 320–400 nm) and visible light (λ = 400–800 nm) can lead to the oxidation of pharmaceutical proteins, which can affect efficiency and promote immunogenicity. However, no concise mechanism has been established for the photo-oxidation of pharmaceutical proteins under near UV and visible light. Here, we show that carboxylic acid buffer-Fe3+ complexes can function as photosensitizers, causing peptide degradation via the formation of various radicals and oxidants. Three pharmaceutical relevant carboxylic acid buffers (citrate, acetate, and succinate) were tested under near UV and visible light. Oxidation reactions were monitored for model peptides containing readily oxidizable amino acids, such as methionine- or leucine-enkephalin and proctolin peptide. Oxidation products were evaluated by RP-HPLC coupled to UV or fluorescent detection and RP-HPLC-MS/MS. Specifically for citrate buffer, the light-induced formation of H2O2, •OH, •CO2–, and formaldehyde was demonstrated. The peptides displayed oxidation of Met, hydroxylation of Tyr and Phe, as well as the formation of novel products from Tyr. Experiments with 18O2 resulted in the incorporation of 18O into various reaction products, consistent with a metal-catalyzed activation of O2 into reactive oxygen species. The addition of EDTA and DTPA did not prevent the oxidation of the peptides and, in some cases, enhanced the oxidation. Our results demonstrate that pharmaceutical buffer-Fe3+ complexes, exposed to UV and visible light, can promote various pathways of oxidation reactions in pharmaceutical formulations.
中文翻译:

近紫外光和可见光诱导药物缓冲液中依赖铁的光降解反应:机理和产品研究
近紫外 (λ = 320–400 nm) 和可见光 (λ = 400–800 nm) 会导致药物蛋白质氧化,从而影响效率并促进免疫原性。然而,对于药物蛋白质在近紫外和可见光下的光氧化,还没有建立简明的机制。在这里,我们表明羧酸缓冲剂-Fe 3+复合物可以作为光敏剂,通过形成各种自由基和氧化剂导致肽降解。在近紫外和可见光下测试了三种药物相关的羧酸缓冲液(柠檬酸盐、醋酸盐和琥珀酸盐)。监测含有易氧化氨基酸的模型肽的氧化反应,例如甲硫氨酸-或亮氨酸-脑啡肽和 proctolin 肽。氧化产物通过 RP-HPLC 结合 UV 或荧光检测和 RP-HPLC-MS/MS 进行评估。特别是对于柠檬酸盐缓冲液,光诱导形成 H 2 O 2 , • OH, • CO 2 –,并证明了甲醛。这些肽表现出 Met 的氧化、Tyr 和 Phe 的羟基化以及来自 Tyr 的新产物的形成。与实验18 ö 2导致掺入18 ø成各种反应产物,为O的金属催化的活化相一致2成活性氧。添加 EDTA 和 DTPA 并不能阻止肽的氧化,并且在某些情况下会增强氧化。我们的结果表明,药物缓冲液-Fe 3+络合物暴露于紫外线和可见光下,可以促进药物制剂中氧化反应的各种途径。
更新日期:2020-11-02
中文翻译:

近紫外光和可见光诱导药物缓冲液中依赖铁的光降解反应:机理和产品研究
近紫外 (λ = 320–400 nm) 和可见光 (λ = 400–800 nm) 会导致药物蛋白质氧化,从而影响效率并促进免疫原性。然而,对于药物蛋白质在近紫外和可见光下的光氧化,还没有建立简明的机制。在这里,我们表明羧酸缓冲剂-Fe 3+复合物可以作为光敏剂,通过形成各种自由基和氧化剂导致肽降解。在近紫外和可见光下测试了三种药物相关的羧酸缓冲液(柠檬酸盐、醋酸盐和琥珀酸盐)。监测含有易氧化氨基酸的模型肽的氧化反应,例如甲硫氨酸-或亮氨酸-脑啡肽和 proctolin 肽。氧化产物通过 RP-HPLC 结合 UV 或荧光检测和 RP-HPLC-MS/MS 进行评估。特别是对于柠檬酸盐缓冲液,光诱导形成 H 2 O 2 , • OH, • CO 2 –,并证明了甲醛。这些肽表现出 Met 的氧化、Tyr 和 Phe 的羟基化以及来自 Tyr 的新产物的形成。与实验18 ö 2导致掺入18 ø成各种反应产物,为O的金属催化的活化相一致2成活性氧。添加 EDTA 和 DTPA 并不能阻止肽的氧化,并且在某些情况下会增强氧化。我们的结果表明,药物缓冲液-Fe 3+络合物暴露于紫外线和可见光下,可以促进药物制剂中氧化反应的各种途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号