Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2020-09-28 , DOI: 10.1016/j.jmgm.2020.107765 Ambrish Kumar Srivastava 1
|
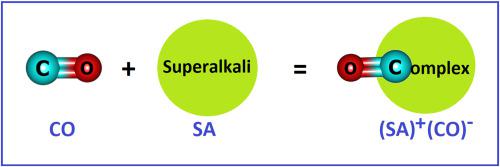
Carbon monoxide (CO) is a toxic gas molecule with no positive electron affinity, which makes it difficult to reduce it into CO¯. In this work, we perform density functional theory (DFT) and quantum theory of atoms in molecule (QTAIM) based studies on the interaction of CO molecule with superalkali (SA) clusters. Our findings suggest that this interaction results in SA(CO) complexes, which are stabilized by purely ionic as well as partially covalent bonds although their binding energy decreases with the increase in the size of SA clusters. In these ionic complexes, the electron is transferred from the SA cluster to the CO molecule. This suggests the single-electron reduction of the CO molecule by interacting with superalkalis. This work may offer some novel insights into the detection and reduction of stable CO molecule and related systems.
中文翻译:

DFT和QTAIM研究超碱还原一氧化碳
一氧化碳(CO)是一种无正电子亲和力的有毒气体分子,因此很难将其还原为CO。在这项工作中,我们基于CO分子与超碱(SA)团簇之间相互作用的研究,进行了分子中原子的密度泛函理论(DFT)和量子理论(QTAIM)。我们的研究结果表明,这种相互作用导致了SA(CO)配合物,尽管它们的结合能随SA簇尺寸的增加而降低,但它们通过纯离子键和部分共价键得以稳定。在这些离子络合物中,电子从SA簇转移到CO分子。这暗示了通过与超碱相互作用,CO分子的单电子还原。这项工作可能会为检测和还原稳定的CO分子及相关系统提供一些新颖的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号