当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intramolecular interaction of B‐MYB is regulated through Ser‐577 phosphorylation
FEBS Letters ( IF 3.5 ) Pub Date : 2020-10-04 , DOI: 10.1002/1873-3468.13940 Eugen Werwein 1 , Abhiruchi Biyanee 1 , Karl‐Heinz Klempnauer 1
FEBS Letters ( IF 3.5 ) Pub Date : 2020-10-04 , DOI: 10.1002/1873-3468.13940 Eugen Werwein 1 , Abhiruchi Biyanee 1 , Karl‐Heinz Klempnauer 1
Affiliation
|
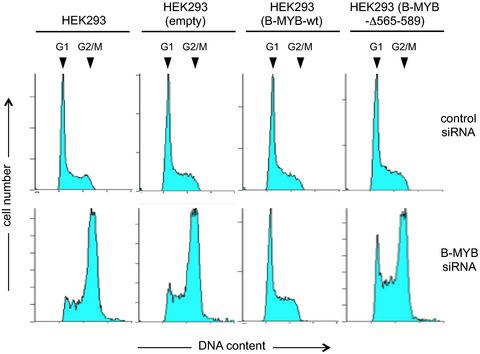
The transcription factor B‐MYB is an important regulator of cell cycle‐related processes that is activated by step‐wise phosphorylation of multiple sites by cyclin‐dependent kinases (CDKs) and conformational changes induced by the peptidylprolyl cis/trans isomerase Pin1. Here, we show that a conserved amino acid sequence around Ser‐577 in the C‐terminal part of B‐MYB is able to interact with the B‐MYB DNA‐binding domain. Phosphorylation of Ser‐577 disrupts this interaction and is regulated by the interplay of CDKs and the phosphatase CDC14B. Deletion of sequences surrounding Ser‐577 hyperactivates the transactivation potential of B‐MYB, decreases its proteolytic stability, and causes cell cycle defects. Overall, we show for the first time that B‐MYB can undergo an intramolecular interaction that is controlled by the phosphorylation state of Ser‐577.
中文翻译:

B-MYB 的分子内相互作用通过 Ser-577 磷酸化调节
转录因子 B-MYB 是细胞周期相关过程的重要调节因子,通过细胞周期蛋白依赖性激酶 (CDK) 对多个位点的逐步磷酸化和肽基脯氨酰顺式/反式异构酶 Pin1 诱导的构象变化激活。在这里,我们表明 B-MYB C 末端部分 Ser-577 周围的保守氨基酸序列能够与 B-MYB DNA 结合域相互作用。Ser-577 的磷酸化破坏了这种相互作用,并受 CDK 和磷酸酶 CDC14B 的相互作用调节。删除 Ser-577 周围的序列会过度激活 B-MYB 的反式激活潜力,降低其蛋白水解稳定性,并导致细胞周期缺陷。全面的,
更新日期:2020-10-04
中文翻译:

B-MYB 的分子内相互作用通过 Ser-577 磷酸化调节
转录因子 B-MYB 是细胞周期相关过程的重要调节因子,通过细胞周期蛋白依赖性激酶 (CDK) 对多个位点的逐步磷酸化和肽基脯氨酰顺式/反式异构酶 Pin1 诱导的构象变化激活。在这里,我们表明 B-MYB C 末端部分 Ser-577 周围的保守氨基酸序列能够与 B-MYB DNA 结合域相互作用。Ser-577 的磷酸化破坏了这种相互作用,并受 CDK 和磷酸酶 CDC14B 的相互作用调节。删除 Ser-577 周围的序列会过度激活 B-MYB 的反式激活潜力,降低其蛋白水解稳定性,并导致细胞周期缺陷。全面的,


























 京公网安备 11010802027423号
京公网安备 11010802027423号