当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cryo‐EM structure of fission yeast tetrameric α‐mannosidase Ams1
FEBS Open Bio ( IF 2.6 ) Pub Date : 2020-09-27 , DOI: 10.1002/2211-5463.12988 Jianxiu Zhang 1, 2 , Ying-Ying Wang 3 , Li-Lin Du 3, 4 , Keqiong Ye 1, 2
FEBS Open Bio ( IF 2.6 ) Pub Date : 2020-09-27 , DOI: 10.1002/2211-5463.12988 Jianxiu Zhang 1, 2 , Ying-Ying Wang 3 , Li-Lin Du 3, 4 , Keqiong Ye 1, 2
Affiliation
|
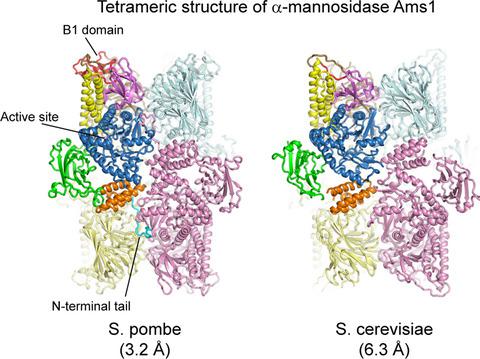
Fungal α‐mannosidase Ams1 and its mammalian homolog MAN2C1 hydrolyze terminal α‐linked mannoses in free oligosaccharides released from misfolded glycoproteins or lipid‐linked oligosaccharide donors. Ams1 is transported by selective autophagy into vacuoles. Here, we determine the tetrameric structure of Ams1 from the fission yeast Schizosaccharomyces pombe at 3.2 Å resolution by cryo‐electron microscopy. Distinct from a low resolution structure of S. cerevisiae Ams1, S. pombe Ams1 has a prominent N‐terminal tail that mediates tetramerization and an extra β‐sheet domain. Ams1 shares a conserved active site with other enzymes in glycoside hydrolase family 38, to which Ams1 belongs, but contains extra N‐terminal domains involved in tetramerization. The atomic structure of Ams1 reported here will aid understanding of its enzymatic activity and transport mechanism.
中文翻译:

裂变酵母四聚体 α-甘露糖苷酶 Ams1 的冷冻电镜结构
真菌 α-甘露糖苷酶 Ams1 及其哺乳动物同源物 MAN2C1 水解从错误折叠的糖蛋白或脂质连接的寡糖供体释放的游离寡糖中的末端 α-连接的甘露糖。Ams1 通过选择性自噬转运到液泡中。在这里,我们通过低温电子显微镜以 3.2 Å 的分辨率从裂殖酵母粟酒裂殖酵母中确定了 Ams1 的四聚体结构。与S. cerevisiae Ams1、S. pombe的低分辨率结构不同Ams1 有一个突出的 N 末端尾部,可介导四聚化和一个额外的 β 折叠结构域。Ams1 与 Ams1 所属的糖苷水解酶家族 38 中的其他酶共享一个保守的活性位点,但包含参与四聚化的额外 N 端结构域。这里报道的 Ams1 的原子结构将有助于理解其酶活性和转运机制。
更新日期:2020-11-04
中文翻译:

裂变酵母四聚体 α-甘露糖苷酶 Ams1 的冷冻电镜结构
真菌 α-甘露糖苷酶 Ams1 及其哺乳动物同源物 MAN2C1 水解从错误折叠的糖蛋白或脂质连接的寡糖供体释放的游离寡糖中的末端 α-连接的甘露糖。Ams1 通过选择性自噬转运到液泡中。在这里,我们通过低温电子显微镜以 3.2 Å 的分辨率从裂殖酵母粟酒裂殖酵母中确定了 Ams1 的四聚体结构。与S. cerevisiae Ams1、S. pombe的低分辨率结构不同Ams1 有一个突出的 N 末端尾部,可介导四聚化和一个额外的 β 折叠结构域。Ams1 与 Ams1 所属的糖苷水解酶家族 38 中的其他酶共享一个保守的活性位点,但包含参与四聚化的额外 N 端结构域。这里报道的 Ams1 的原子结构将有助于理解其酶活性和转运机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号