当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile Synthesis of 2‐Fluorobenzofurans: 5‐endo‐trig Cyclization of β,β‐Difluoro‐o‐hydroxystyrenes
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-09-26 , DOI: 10.1002/hlca.202000159 Ryutaro Morioka 1 , Takeshi Fujita 1 , Junji Ichikawa 1
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-09-26 , DOI: 10.1002/hlca.202000159 Ryutaro Morioka 1 , Takeshi Fujita 1 , Junji Ichikawa 1
Affiliation
|
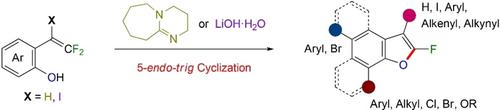
Efficient synthetic methods were established for obtaining 2‐fluorobenzofurans involving various substituents. Upon being treated with 1,8‐diazabicyclo[5.4.0]undec‐7‐ene under microwave irradiation, the α‐unsubstituted β,β‐difluoro‐o‐hydroxystyrenes underwent nucleophilic 5‐endo‐trig cyclization to afford the corresponding 2‐fluorobenzofurans in high yields. Furthermore, 2‐fluoro‐3‐iodobenzofuran was successfully synthesized, and its transformation to various 3‐substituted 2‐fluorobenzofurans was demonstrated.
中文翻译:

2-氟苯并呋喃的简便合成:β,β-二氟-邻羟基苯乙烯的5-内-trig环化
建立了获得涉及各种取代基的2-氟苯并呋喃的有效合成方法。在微波辐射下用1,8-二氮杂双环[5.4.0]十一碳-7-烯处理后,α-未取代的β,β-二氟-邻羟基苯乙烯进行亲核的5-内-trig环化,得到相应的2 - n-高产率的氟苯并呋喃。此外,成功地合成了2-氟-3-碘苯并呋喃,并证明了其向各种3-取代的2-氟苯并呋喃的转化。
更新日期:2020-11-25
中文翻译:

2-氟苯并呋喃的简便合成:β,β-二氟-邻羟基苯乙烯的5-内-trig环化
建立了获得涉及各种取代基的2-氟苯并呋喃的有效合成方法。在微波辐射下用1,8-二氮杂双环[5.4.0]十一碳-7-烯处理后,α-未取代的β,β-二氟-邻羟基苯乙烯进行亲核的5-内-trig环化,得到相应的2 - n-高产率的氟苯并呋喃。此外,成功地合成了2-氟-3-碘苯并呋喃,并证明了其向各种3-取代的2-氟苯并呋喃的转化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号