当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
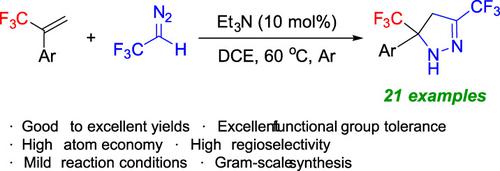
Et3N‐Catalyzed Cycloaddition Reactions of α‐(Trifluoromethyl)styrenes with 2,2,2‐Trifluorodiazoethane to Access Bis(trifluoromethyl)‐Substituted Pyrazolines
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-09-26 , DOI: 10.1002/cjoc.202000480 Chunmei Li 1 , Xuxue Zhang 2 , Jingjing He 1 , Sixue Xu 1 , Song Cao 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-09-26 , DOI: 10.1002/cjoc.202000480 Chunmei Li 1 , Xuxue Zhang 2 , Jingjing He 1 , Sixue Xu 1 , Song Cao 1
Affiliation
|
A novel and practical method for the synthesis of 3,5‐bis(trifluoromethyl)‐4,5‐dihydro‐1H‐pyrazoles by [3+2] cycloaddition reactions of α‐(trifluoromethyl)styrenes with 2,2,2‐trifluorodiazoethane (CF3CHN2) has been developed. The cyclization reaction proceeds smoothly in the presence of a catalytic amount of Et3N, affording a variety of bis(trifluoromethyl)‐substituted 2‐pyrazolines in good to excellent yields. This method also exhibits a broad substrate scope and tolerates various functional groups.
中文翻译:

Et3N催化的α-(三氟甲基)苯乙烯与2,2,2-三氟重氮乙烷的环加成反应获得双(三氟甲基)取代的吡唑啉
通过α-(三氟甲基)苯乙烯与2,2,2-的[3 + 2]环加成反应合成3,5-双(三氟甲基)-4,5-二氢-1H-吡唑的新颖实用方法已经开发出三氟重氮乙烷(CF 3 CHN 2)。在催化量的Et 3 N的存在下,环化反应平稳进行,从而提供了多种双(三氟甲基)取代的2-吡唑啉,收率高至优异。该方法还表现出广泛的底物范围,并能耐受各种官能团。
更新日期:2020-09-26

中文翻译:

Et3N催化的α-(三氟甲基)苯乙烯与2,2,2-三氟重氮乙烷的环加成反应获得双(三氟甲基)取代的吡唑啉
通过α-(三氟甲基)苯乙烯与2,2,2-的[3 + 2]环加成反应合成3,5-双(三氟甲基)-4,5-二氢-1H-吡唑的新颖实用方法已经开发出三氟重氮乙烷(CF 3 CHN 2)。在催化量的Et 3 N的存在下,环化反应平稳进行,从而提供了多种双(三氟甲基)取代的2-吡唑啉,收率高至优异。该方法还表现出广泛的底物范围,并能耐受各种官能团。



























 京公网安备 11010802027423号
京公网安备 11010802027423号