Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (+)-Petromyroxol, (−)-iso-Petromyroxol, and Possible Diastereomers
ACS Omega ( IF 4.1 ) Pub Date : 2020-09-24 , DOI: 10.1021/acsomega.0c03674 Venkannababu Mullapudi 1, 2 , Iram Ahmad 1 , Sibadatta Senapati 1, 2 , Chepuri V. Ramana 1, 2
ACS Omega ( IF 4.1 ) Pub Date : 2020-09-24 , DOI: 10.1021/acsomega.0c03674 Venkannababu Mullapudi 1, 2 , Iram Ahmad 1 , Sibadatta Senapati 1, 2 , Chepuri V. Ramana 1, 2
Affiliation
|
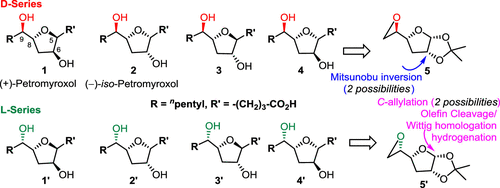
The total synthesis of (+)-petromyroxol (1) and its seven diastereomers including the (−)-iso-petromyroxol (2) is described. The employed strategy involves the use of easily available C5-epimeric epoxides 5 and 5′ and nonselective anomeric C1-allylation, proceeding with or without inversion at C2, thereby giving the possibility of synthesizing all possible diastereomers. Extensive two-dimensional (2D) NMR analyses of all eight diastereomers have been carried out to assign the chemical shifts of the central carbons and the corresponding attached hydrogens and to learn how the C/H-chemical shifts of the tetrahydrofuran ring were influenced by the adjacent centers.
中文翻译:

(+)-Petromyroxol,(-)- iso- Petromyroxol和可能的非对映异构体的全合成
描述了(+)-邻苯二酚(1)及其七个非对映异构体的全合成,包括(-)-异-邻苯二酚(2)。所采用的策略涉及使用容易获得的C5-表异构体环氧化物5和5'和非选择性异头异构体C1-烯丙基化,在C2处进行或不进行转化,从而提供了合成所有可能的非对映异构体的可能性。已经对所有8个非对映异构体进行了广泛的二维(2D)NMR分析,以确定中心碳和相应的附着氢的化学位移,并研究四氢呋喃环的C / H化学位移如何受到氢的影响。相邻的中心。
更新日期:2020-10-06
中文翻译:

(+)-Petromyroxol,(-)- iso- Petromyroxol和可能的非对映异构体的全合成
描述了(+)-邻苯二酚(1)及其七个非对映异构体的全合成,包括(-)-异-邻苯二酚(2)。所采用的策略涉及使用容易获得的C5-表异构体环氧化物5和5'和非选择性异头异构体C1-烯丙基化,在C2处进行或不进行转化,从而提供了合成所有可能的非对映异构体的可能性。已经对所有8个非对映异构体进行了广泛的二维(2D)NMR分析,以确定中心碳和相应的附着氢的化学位移,并研究四氢呋喃环的C / H化学位移如何受到氢的影响。相邻的中心。



























 京公网安备 11010802027423号
京公网安备 11010802027423号