当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Role of Carbonate in Catalytic Oxidations
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-09-25 , DOI: 10.1021/acs.accounts.0c00344 Shanti Gopal Patra 1 , Amir Mizrahi 2 , Dan Meyerstein 1, 3
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-09-25 , DOI: 10.1021/acs.accounts.0c00344 Shanti Gopal Patra 1 , Amir Mizrahi 2 , Dan Meyerstein 1, 3
Affiliation
|
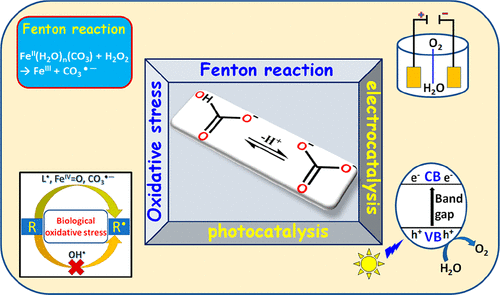
CO2, HCO3–, and CO32– are present in all aqueous media at pH > 4 if no major effort is made to remove them. Usually the presence of CO2/HCO3–/CO32– is either forgotten or considered only as a buffer or proton transfer catalyst. Results obtained in the last decades point out that carbonates are key participants in a variety of oxidation processes. This was first attributed to the formation of carbonate anion radicals via the reaction OH• + CO32– → CO3•– + OH–. However, recent studies point out that the involvement of carbonates in oxidation processes is more fundamental. Thus, the presence of HCO3–/CO32– changes the mechanisms of Fenton and Fenton-like reactions to yield CO3•– directly even at very low HCO3–/CO32– concentrations. CO3•– is a considerably weaker oxidizing agent than the hydroxyl radical and therefore a considerably more selective oxidizing agent. This requires reconsideration of the sources of oxidative stress in biological systems and might explain the selective damage induced during oxidative stress. The lower oxidation potential of CO3•– probably also explains why not all pollutants are eliminated in many advanced oxidation technologies and requires rethinking of the optimal choice of the technologies applied. The role of percarbonate in Fenton-like processes and in advanced oxidation processes is discussed and has to be re-evaluated. Carbonate as a ligand stabilizes transition metal complexes in uncommon high oxidation states. These high-valent complexes are intermediates in electrochemical water oxidation processes that are of importance in the development of new water splitting technologies. HCO3– and CO32– are also very good hole scavengers in photochemical processes of semiconductors and may thus become key participants in the development of new processes for solar energy conversion. In this Account, an attempt to correlate these observations with the properties of carbonates is made. Clearly, further studies are essential to fully uncover the potential of HCO3–/CO32– in desired oxidation processes.
中文翻译:

碳酸盐在催化氧化中的作用
如果不做很大的努力来除去pH> 4,则所有水性介质中都会存在CO 2,HCO 3 –和CO 3 2 –。通常,CO 2 / HCO 3 – / CO 3 2–的存在被遗忘或仅被视为缓冲剂或质子转移催化剂。在过去的几十年中获得的结果表明,碳酸盐是各种氧化过程的关键参与者。这首先归因于通过OH • + CO 3 2– →CO 3 •– + OH –反应形成的碳酸根阴离子自由基。。但是,最近的研究指出,碳酸盐参与氧化过程更为根本。因此,即使在非常低的HCO 3 – / CO 3 2 –浓度下,HCO 3 – / CO 3 2–的存在也会改变Fenton和类似Fenton的反应机理,直接产生CO 3 •–。CO 3 •–是比羟基自由基弱得多的氧化剂,因此是选择性更高的氧化剂。这需要重新考虑生物系统中氧化应激的来源,并可能解释氧化应激过程中引起的选择性损伤。一氧化碳的氧化电位较低3 •–可能还解释了为什么在许多先进的氧化技术中不能消除所有污染物,并且需要重新考虑所应用技术的最佳选择。讨论过碳酸盐在类似Fenton的过程中和在高级氧化过程中的作用,必须重新评估。碳酸盐作为配体可将过渡金属络合物稳定在罕见的高氧化态下。这些高价络合物是电化学水氧化过程中的中间体,在新的水分解技术的开发中很重要。HCO 3 –和CO 3 2–它们在半导体的光化学工艺中也是非常好的空穴清除剂,因此可能成为开发太阳能转化新工艺的关键参与者。在该帐户中,试图将这些观察结果与碳酸盐的性质联系起来。显然,进一步的研究是必要的充分揭露HCO的电位3 - / CO 3 2-中所需的氧化过程。
更新日期:2020-10-21
中文翻译:

碳酸盐在催化氧化中的作用
如果不做很大的努力来除去pH> 4,则所有水性介质中都会存在CO 2,HCO 3 –和CO 3 2 –。通常,CO 2 / HCO 3 – / CO 3 2–的存在被遗忘或仅被视为缓冲剂或质子转移催化剂。在过去的几十年中获得的结果表明,碳酸盐是各种氧化过程的关键参与者。这首先归因于通过OH • + CO 3 2– →CO 3 •– + OH –反应形成的碳酸根阴离子自由基。。但是,最近的研究指出,碳酸盐参与氧化过程更为根本。因此,即使在非常低的HCO 3 – / CO 3 2 –浓度下,HCO 3 – / CO 3 2–的存在也会改变Fenton和类似Fenton的反应机理,直接产生CO 3 •–。CO 3 •–是比羟基自由基弱得多的氧化剂,因此是选择性更高的氧化剂。这需要重新考虑生物系统中氧化应激的来源,并可能解释氧化应激过程中引起的选择性损伤。一氧化碳的氧化电位较低3 •–可能还解释了为什么在许多先进的氧化技术中不能消除所有污染物,并且需要重新考虑所应用技术的最佳选择。讨论过碳酸盐在类似Fenton的过程中和在高级氧化过程中的作用,必须重新评估。碳酸盐作为配体可将过渡金属络合物稳定在罕见的高氧化态下。这些高价络合物是电化学水氧化过程中的中间体,在新的水分解技术的开发中很重要。HCO 3 –和CO 3 2–它们在半导体的光化学工艺中也是非常好的空穴清除剂,因此可能成为开发太阳能转化新工艺的关键参与者。在该帐户中,试图将这些观察结果与碳酸盐的性质联系起来。显然,进一步的研究是必要的充分揭露HCO的电位3 - / CO 3 2-中所需的氧化过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号